Categories
Change Password!
Reset Password!


Atogepant, an oral calcitonin gene-related peptide (CGRP) antagonist, is used for the management of migraine. [1] In September 2021, FDA approved atogepant as a preventive therapy for episodic migraine in adults.
Atogepant, an oral calcitonin gene-related peptide (CGRP) antagonist, is used for the management of migraine. [1] In September 2021, FDA approved atogepant as a preventive therapy for episodic migraine in adults. [2]
Pharmacological Class: CGRP receptor antagonist (Gepants) [3]
It is used for the prevention of episodic migraine in adults. [1]
CGRP, a 37-amino acid peptide, is localized in the peripheral and central sensory nervous systems. It serves as a neurotransmitter and a potent vasodilator. It mediates its biological effects through its interactions with the CGRP receptor. [3] The α-isoform of CGRP (expressed in primary sensory neurons) is involved in pathogenesis of migraine. [1]
The levels of CGRP are acutely increased during migraine attacks. Along with its vasodilatory effects, CGRP appears to be a pronociceptive factor that regulates neuronal excitability for facilitating pain responses. Atogepant, an antagonist of CGRP receptor, competes with CGRP for occupancy at these receptors. This, in turn, prevents CGRP actions and its ability to trigger and perpetuate migraine. [1]
Absorption
After oral administration, the time to peak plasma concentration is about two-three hours. It displays dose-proportional pharmacokinetics up to about three-fold its suggested maximum dosage. Atogepant's pharmacokinetics are not considerably affected by co-administration with food.
Volume of distribution
The mean apparent volume of distribution is approximately 292 L.
Protein binding
It is extensively (~95.3%) protein-bound in plasma.
Metabolism
Atogepant's metabolism is mediated primarily via CYP3A4. In the plasma, the most prevalent circulating compounds are atogepant itself and glucuronide conjugate metabolite (M23), encompassing about 75% and 15% of the given dose, respectively, with at least ten other metabolites detected in feces representing <10% of the given dosage.
Route of elimination
Atogepant is eliminated predominantly via CYP3A4 metabolism. After a single oral dose of radiolabeled atogepant to healthy males, about 5% of given dose was recovered as unchanged parent drug in urine and 42% of given dose was recovered as unchanged parent drug in feces. Notably, 81% of radioactivity was recovered in feces, with only 8% of radioactivity recovered in urine.
Half-life
After oral administration, the elimination half-life is about eleven hours.
Clearance
The mean apparent oral clearance is about 19 L/h. [1]
None
OATP Inhibitors
Concomitant administration of atogepant with single-dose rifampin (organic anion transporting polypeptides [OATP] inhibitor) led to a considerable rise in the exposure of atogepant in healthy people. Thus, atogepant's suggested dose with concomitant utilization of OATP inhibitors (cyclosporine) is 10 mg or 30 mg once daily.
CYP3A4 Inducers
Concomitant administration of atogepant with steady-state rifampin (a strong CYP3A4 inducer) elicited a substantial drop in the exposure of atogepant in healthy people. Concomitant administration of moderate inducers of CYP3A4 and atogepant can also cause reduced exposure of atogepant.
The suggested dose of atogepant with concomitant utilization of moderate or strong CYP3A4 inducers (etravirine, efavirenz, St. John’s wort, phenytoin, carbamazepine, rifampin) is 30 mg or 60 mg once daily. There is no need to modify atogepant's dose with concomitant usage of weak CYP3A4 inducers
CYP3A4 Inhibitors
Concomitant administration of atogepant with itraconazole (a strong CYP3A4 inhibitor) led to a considerable rise in the exposure of atogepant in healthy people. The suggested dosage of atogepant with concomitant usage of strong CYP3A4 inhibitors (such as clarithromycin, ketoconazole, itraconazole) is 10 mg once daily. There is no need to adjust atogepant's dosage with concomitant utilization of weak or moderate CYP3A4 inhibitors. [4]
The most commonly reported side effects are:
Atogepant reduces monthly migraine days in migraine patients
A phase 2b/3 double-blind randomized trial demonstrated that all the doses of oral atogepant (10 mg once daily, 30 mg once daily, 60 mg once daily, 30 mg twice daily, 60 mg twice daily) were linked with a considerable reduction in the monthly migraine days over twelve weeks in comparison with placebo. It was safe and showed good tolerability over twelve weeks, supporting its phase III development for the preventive therapy of migraine.
This study was carried out to determine the effectiveness, safety, and tolerability of orally administered atogepant to prevent episodic migraine in adults (age18–75 years) having a history (≥one year) of migraine and 4–14 migraine days per month. Overall, 825 participants were randomized to get placebo (n=186) or atogepant 10 mg once daily (n=93), 30 mg once daily (n=183), 60 mg once daily (n=186), 30 mg twice daily (n=86), or 60 mg twice daily (n=91), in the matching capsules.
An alteration from baseline in the monthly migraine days across twelve weeks of therapy utilizing a modified intention-to-treat approach was the major endpoint. For evaluating tolerability and safety, adverse events were recorded. The primary efficacy assessment incorporated 795 people. Across the twelve-week treatment period, all the five atogepant groups illustrated substantial least-squares mean (standard error) alteration from the baseline in mean monthly migraine days when compared to placebo. (Fig.1)
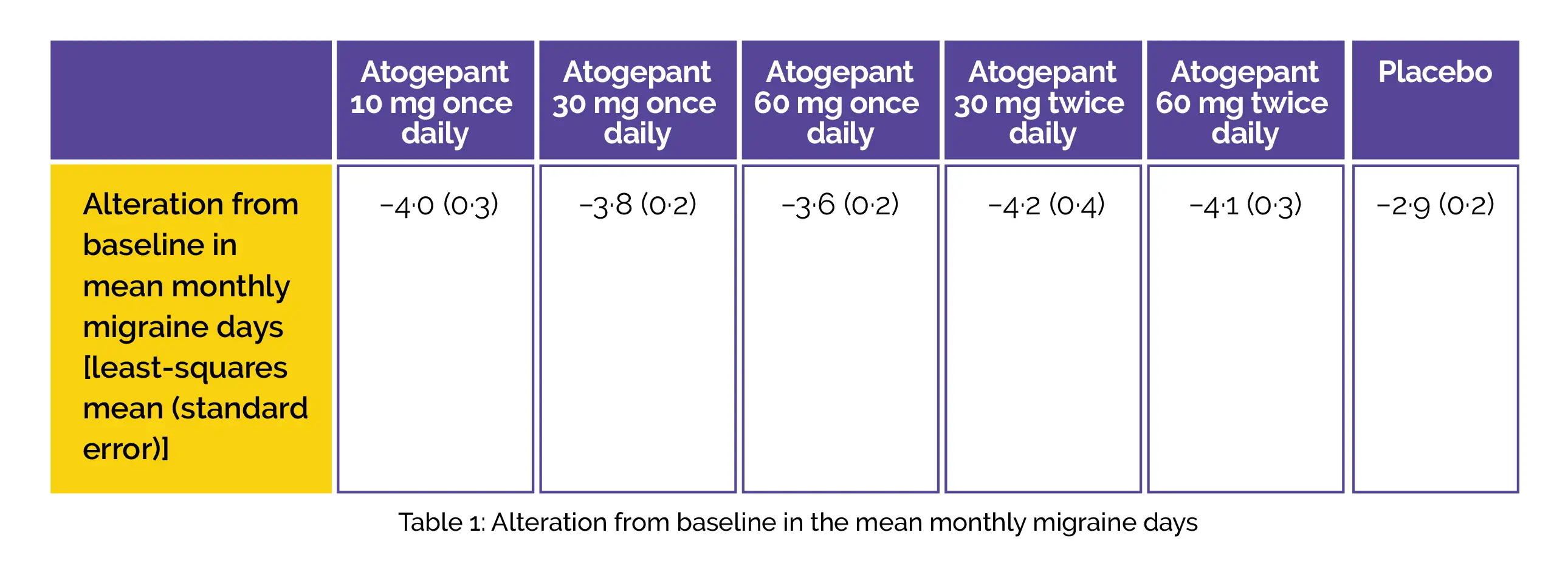
Nausea and fatigue were the most commonly noted treatment-emergent adverse events (TEAEs) across all the groups. The frequency of TEAEs was reported to range from 26% (24/91) for 60 mg twice daily to 18% (17/93) for 10 mg once daily when compared to 16% (30/186) for the placebo-recipients.
Overall, 7 people experienced 8 serious TEAEs (2 people each in placebo, 30 mg once-daily, and 60 mg once-daily groups, and 1 person in 10 mg once-daily group). The TEAEs resulting in study discontinuation were noted in 5% (33/639) atogepant recipients and 3% (5/186) of those randomized to the placebo group. All the severe TEAEs were not related to treatment. [5]
1. Atogepant. Drug Bank. Accession Number DB16098. Available online from: https://go.drugbank.com/drugs/DB16098. [Last accessed on: 24 December 2021]
2. Atogepant receives FDA approval for the preventive treatment of episodic migraine in adults. Available online from: https://americanheadachesociety.org/news/atogepant-receives-fda-approval-for-the-preventive-treatment-of-episodic-migraine-in-adults/ [Last accessed on: 24 December 2021]
3. Rivera-Mancilla E, Villalón CM, MaassenVanDenBrink A. CGRP inhibitors for migraine prophylaxis: a safety review. Expert Opinion on Drug Safety. 2020 Oct 2;19(10):1237-50.
4. Atogepant. FDA LABEL. Available online from:https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215206Orig1s000lbl.pdf [Last accessed on: 24 December 2021]
5.Goadsby PJ, Dodick DW, Ailani J, Trugman JM, Finnegan M, Lu K et al. Safety, tolerability, and efficacy of orally administered atogepant for the prevention of episodic migraine in adults: a double-blind, randomised phase 2b/3 trial. The Lancet Neurology. 2020 Sep 1;19(9):727-37.
Comments (0)