Categories
Change Password!
Reset Password!


Doxepin is a dibenzoxepine derived-tricyclic antidepressant employed in the management of major depressive disorder (MDD), anxiety, and insomnia.
Doxepin is a dibenzoxepine derived-tricyclic antidepressant employed in the management of major depressive disorder (MDD), anxiety, and insomnia. [1,2] Its approval by the FDA for MDD or anxiety dates back to 1969, while it gained FDA approval for insomnia in 2010. [1-3]
Doxepin acts by suppression of serotonin (5-HT) and norepinephrine (NE) reuptake within synaptic clefts in the central nervous system (CNS), thus boosting brain levels of these neurotransmitters. [1] This tricyclic medication also exhibits antagonist effects on histaminic, muscarinic, and alpha-adrenergic receptors. [2,4]
Pharmacological Class: Tricyclic antidepressants [2]
Doxepin is a psychotropic medication employed in the management of depression, anxiety, insomnia, and bipolar (manic-depressive) disorder. [5]
Doxepin's antidepressant action is primarily linked to the inhibition of biogenic amine reuptake in the CNS, particularly NE and 5-HT at synaptic nerve terminals. This inhibition elevates monoamines levels in the synaptic cleft, subsequently enhancing action at the post-synaptic neuron receptor sites. Additionally, there is a suggestion that doxepin may desensitize both 5-HT 1A receptors and beta-adrenergic receptors.
The dearth of dopamine transporters in the frontal cortex is recognized, and the predominant inhibition of dopamine transport in this area is attributed to the influence of NE reuptake. Consequently, the suggested effect of Doxepin on the frontal cortex is to enhance dopamine neurotransmission in this specific area. [5] When administered at low doses (<10 mg), Doxepin exhibits a strong affinity for the histamine H1 receptor while having minimal impact on serotonergic or adrenergic receptors, thereby qualifying as a selective histamine H1 antagonist.
The standard antidepressant dose exceeds 75 mg per day. At low doses (<10 mg), doxepin predominantly targets histamine H1 receptors without significant impact on serotonergic or adrenergic receptors, exhibiting selectivity as a histamine H1 antagonist. The hypothesis suggests that blocking histamine H1 receptors during a circadian cycle, when histamine release and wakefulness decrease, may facilitate and sustain sleep. [4]
For insomnia:
For anxiety/depression:
(a) In adults
(b) In geriatrics
Absorption
Doxepin demonstrates moderate absorption upon oral ingestion, having a bioavailability of 30%. Its median peak concentration varies from 8.8-45.8 ng/ml and is achieved 3.5 hours after the initial administration. Its absorption is escalated when administered alongside a high-fat meal.
Volume of Distribution
The reported mean apparent volume of distribution is 20 L/kg.
Protein binding
According to equilibrium dialysis, Doxepin illustrates an average protein binding of 75.5%, while desmethyldoxepin exhibits a protein binding of 76%.
Metabolism
Doxepin undergoes substantial metabolic transformation to produce N-desmethyldoxepin, a biologically active metabolite, along with other inactive metabolites. The first-pass metabolism contributes to 55-87% of the administered dose. Subsequently, secondary metabolism takes place as N-desmethyldoxepin is transformed into its glucuronide form.
The primary metabolic enzymes engaged in the conversion of Doxepin include members of the cytochrome P450 family, specifically CYP2C19 and CYP2D6, with minor participation from CYP1A2 and CYP2C9.
Route of elimination
The excretion pattern of Doxepin is characterized by two phases. It is predominantly eliminated through urine in the form of glucuronide conjugates. Less than three percent of the administered Doxepin dose is eliminated in the urine as either Nordoxepin or the parent compound.
Half-life
The reported mean elimination half-life is 15 hours.
Clearance
In healthy individuals, the average total apparent plasma clearance of a 50 mg oral dosage of Doxepin is 0.93 L per hour per kilogram. [5]
The most commonly encountered treatment-emergent adverse reactions are:
Uncommon yet potentially serious side effects encompass thoughts of suicide and behaviors, as well as sudden onset of acute glaucoma. [1-3]
(A) Comparison of efficacy between Doxepin and Zolpidem for insomnia
In a clinical investigation involving 120 participants, the impact of administering Doxepin at a dosage of 6 mg/day and Zolpidem at a dosage ranging from 5–10 mg/day orally were examined concerning their influence on sleep composition and cognitive functioning in individuals diagnosed with insomnia disorder. Following the 8-week treatment period, improvements were observed in total sleep time (TST) and wake after sleep onset (WASO) values within the Doxepin group (TST: 378.9 ± 21.9 min, WASO: 80.3 ± 21.4 min) in comparison to the Zolpidem group (TST: 333.2 ± 24.2 min, WASO: 132.9 ± 26.5 min).
The Doxepin group exhibited a longer sleep onset latency (28.2 ± 5.6 min) than the Zolpidem group (20.3 ± 4.7 min). It also displayed higher sleep efficiency (77.8 ± 4.2%) compared to the Zolpidem group (68.6 ± 5.0%). Additionally, the Doxepin group achieved a lower Pittsburgh Sleep Quality Index (PSQI) score (6.1 ± 1.1) as opposed to the Zolpidem group (7.9 ± 1.9). Nevertheless, a higher incidence of treatment-related adverse events was experienced in the Doxepin group (23.3%) in comparison with the Zolpidem group (13.3%). Hence, both Doxepin and Zolpidem proved efficient in enhancing sleep quality, but their effects varied. Doxepin demonstrated a superior improvement in executive function compared to Zolpidem in individuals diagnosed with insomnia disorder. [8]
(B) Pediatric evaluation of Doxepin effectiveness in insomnia
In another investigation, the effectiveness of Doxepin was examined in 29 insomnia-affected pediatrics (ranging from 2 to 17 years old). The therapy began with a median initial dose of 2 mg and was gradually elevated to a median maintenance dosage of 10 mg. The typical length of follow-up was 6.5 ± 3.5 months. Out of 29 subjects, 4 patients (13.8%) discontinued the usage of Doxepin due to either ineffective results or adverse reactions.
Among the remaining participants, 8 patients (27.6%) exhibited significant improvement in their insomnia, 8 (27.6%) demonstrated moderate improvement, 10 (34.5%) showed mild improvement, and 3 (10.3%) experienced minimal to no enhancement while on Doxepin treatment, as shown in Figure 1:
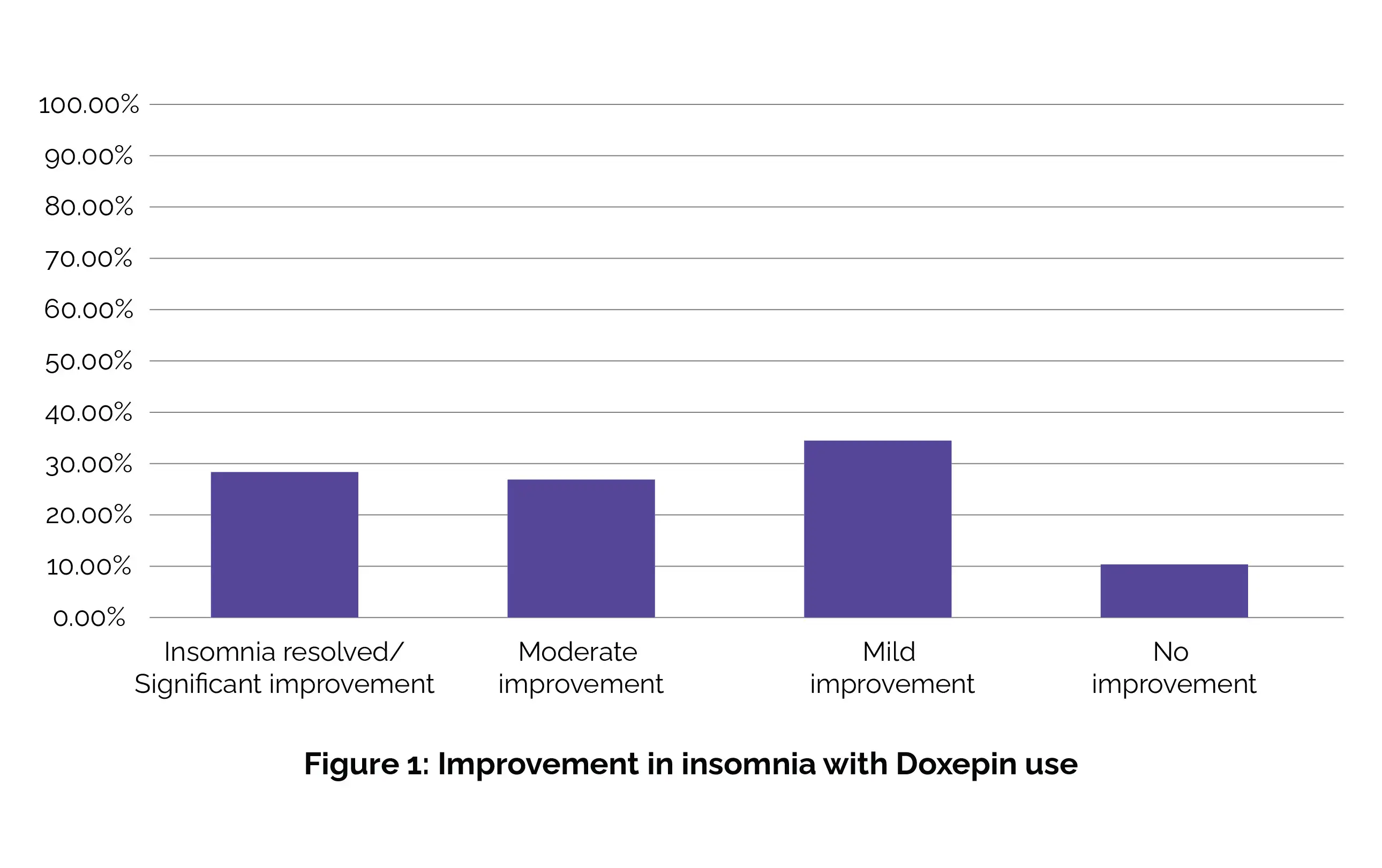
Adverse effects, specifically behavioral side effects such as aggression and enuresis, were reported in only 2 patients (6.9%). Hence, the findings of this study indicate that administering Doxepin at a low dose is both efficacious and well-tolerated in pediatric patients dealing with insomnia. [9]
(C) Low-dose Doxepin treatment for generalized anxiety disorder (GAD)
In a study carried out by Zhang M et al, low-dose Doxepin demonstrated positive clinical effectiveness and safety for GAD management. It proved beneficial in alleviating anxiety in patients with GAD and exhibited effects on neuroendocrine systems, as well as metabolic activity related to serum glucose and lipids. Overall, 49 Chinese patients (twenty males and twenty-nine females) with GAD were assigned randomly to receive low-dose Doxepin (6.25 mg–12.5 mg per day).
Following 12 weeks of administering the drug, the mean Hamilton anxiety rating scale (HAMA) exhibited a decrease from 19.50 ± 1.22 to 8.50 ± 3.61 with low-dose Doxepin treatment. Notably, the serum levels of free triiodothyronine (4.78 ± 0.51 vs 5.15 ± 0.52 pg/mL), adrenocorticotropic hormone (ACTH) (4.33 ± 2.14 vs 6.12 ± 3.02 pmol/L), total cholesterol (4.55 ± 1.01 vs 5.93 ± 1.66 mmol/L), low-density lipoprotein cholesterol (LDLC) (2.43 ± 0.88 vs 3.76 ± 1.25 mmol/L), fasting blood glucose (5.06 ± 0.43 vs 5.78 ± 0.81 mmol/L), and triglyceride(1.69 ± 1.51 vs 3.39 ± 2.86 mmol/L) were significantly higher than pre-treatment levels. However, there were no vital differences in body weight (62.00 ± 7.45 vs 64.00 ± 6.44 kg) and body mass index (23.70 ± 2.35 vs 24.48 ± 2.11 kg/m2) after the treatment. [10]
(D) Low-dose Doxepin for relieving comorbid insomnia with anxiety disorders
In a randomized parallel-group trial, 78 individuals with comorbid insomnia and anxiety disorders were randomly allocated into two groups. The first group (39 cases) received Citalopram at a daily dosage of 20 mg, while the second group (39 cases) was administered Doxepin at a dosage ranging from 6.25 to 12.5 mg per day for twelve weeks.
At three assessment time-points of 4, 8, and 12 weekends, it was observed that both Citalopram and Doxepin groups exhibited a reduction in PSQI scores and the six factor scores of PSQI, with statistical significance (P < 0.05). Additionally, the anxiety levels in both groups remarkably improved with the observed enhancement in sleep quality (P < 0.05). At the 8th weekend (P < 0.01) and 12th weekend (P < 0.001), the Doxepin group demonstrated a shorter sleep onset latency and enhanced subjective sleep quality compared to the Citalopram group.
Importantly, no profound distinction was witnessed in the occurrence of adverse events between the two arms (P = 0.404). These results suggest that the use of low-dose Doxepin is efficacious in treating patients with co-existing insomnia and anxiety disorders. [11]
1. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. Doxepin. [Updated 2018 Jan 8]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK548035/. Bookshelf ID: NBK548035, PMID: 31643366.
2. Almasi A, Meza CE. Doxepin. [Updated 2022 Jan 3]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK542306/. Bookshelf ID: NBK542306, PMID: 31194446.
3. Silenor. FDA Label. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/022036s006lbl.pdf [Last Accessed on: 20 Jan 2024]
4. Yeung WF, Chung KF, Yung KP, Ng TH. Doxepin for insomnia: a systematic review of randomized placebo-controlled trials. Sleep Medicine Reviews. 2015 Feb;19:75-83.
5. Doxepin. DrugBank. Drug Bank Accession Number: DB01142. Available from: https://go.drugbank.com/drugs/DB01142. [Last Accessed on: 24 Jan 2024]
6. Doxepin. Medscape. Available from: https://reference.medscape.com/drug/silenor-doxepin-342940 [Last Accessed on: 24 Jan 2024]
7. Lie JD, Tu KN, Shen DD, Wong BM. Pharmacological Treatment of Insomnia. Pharmacy and Therapeutics. 2015 Nov;40(11):759-71.
8. Yu Z, Han L, Pan Y, Liu W, Ren L, Xu Y et al. Doxepin is more effective than zolpidem in improving executive function in patients with insomnia disorder. Sleep and Breathing. 2023 Dec 20:1-6.
9. Shah Y, Stringel V, Pavkovic I, Kothare SV. Doxepin in children and adolescents with symptoms of insomnia: a single-center experience. Journal of Clinical Sleep Medicine. 2020 May 15;16(5):743–7.
10. Zhang M, Huang F, Jiang F, Mai M, Guo X, Zhang Y et al. Clinical efficacy and safety of low-dose doxepin in Chinese patients with generalized anxiety disorder: A before-after study. Medicine (Baltimore). 2022 Oct 21;101(42): e31201.
11. Chang F, Hu X, Duan L, Xu Y, Wu J, Zu H. Study of the Clinical Effect and Safety of the Low-Dose Doxepin in the Treatment of Comorbid Insomnia Patients with Anxiety Disorders. Advances in Clinical Medicine. 2019 Jan 1;09(04):619–29.
Comments (0)