Categories
Change Password!
Reset Password!


Tigecycline is a semisynthetic antimicrobial agent from the glycylcycline class. It was developed to tackle the rising problem of antibiotic resistance in bacteria, including Staphylococcus aureus.
Tigecycline is a semisynthetic antimicrobial agent from the glycylcycline class. It was developed to tackle the rising problem of antibiotic resistance in bacteria, including Staphylococcus aureus. It is primarily used for the management of polymicrobial infections induced by multidrug-resistant bacteria. [1,2]
The Food and Drug Administration (FDA) provided its fast-track approval on June 17, 2005. [2] Tigecycline is structurally similar to minocycline. The molecular modifications have broadened its activity spectrum and made it less prone to resistance when compared to other tetracycline antibiotic agents. [3]
Pharmacological Class: Glycylcycline class of antibiotics [1]
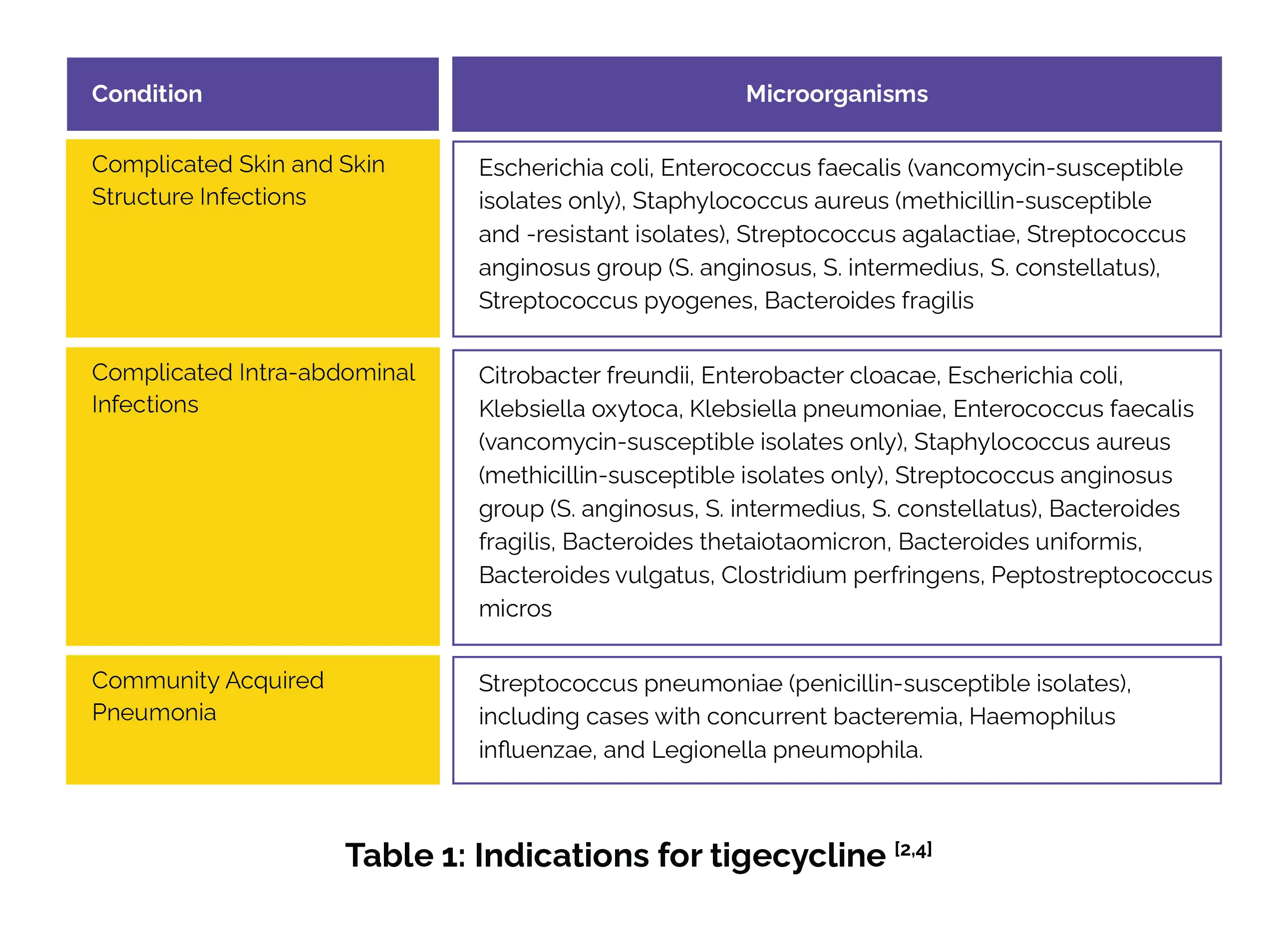
It is used for addressing infections elicited by susceptible strains of the listed microorganisms in the following conditions (Table 1):
Tigecycline has a broad spectrum of activity and works by targeting bacterial protein synthesis. It binds to the 30S ribosomal subunit. This action blocks amino acyl tRNA from entering the ribosome's A site. This action stops amino acids from being added to growing peptide chains, halting protein production. Tigecycline is generally considered bacteriostatic, but it has shown bactericidal activity against Streptococcus pneumoniae and Legionella pneumophila isolates. [3,4]
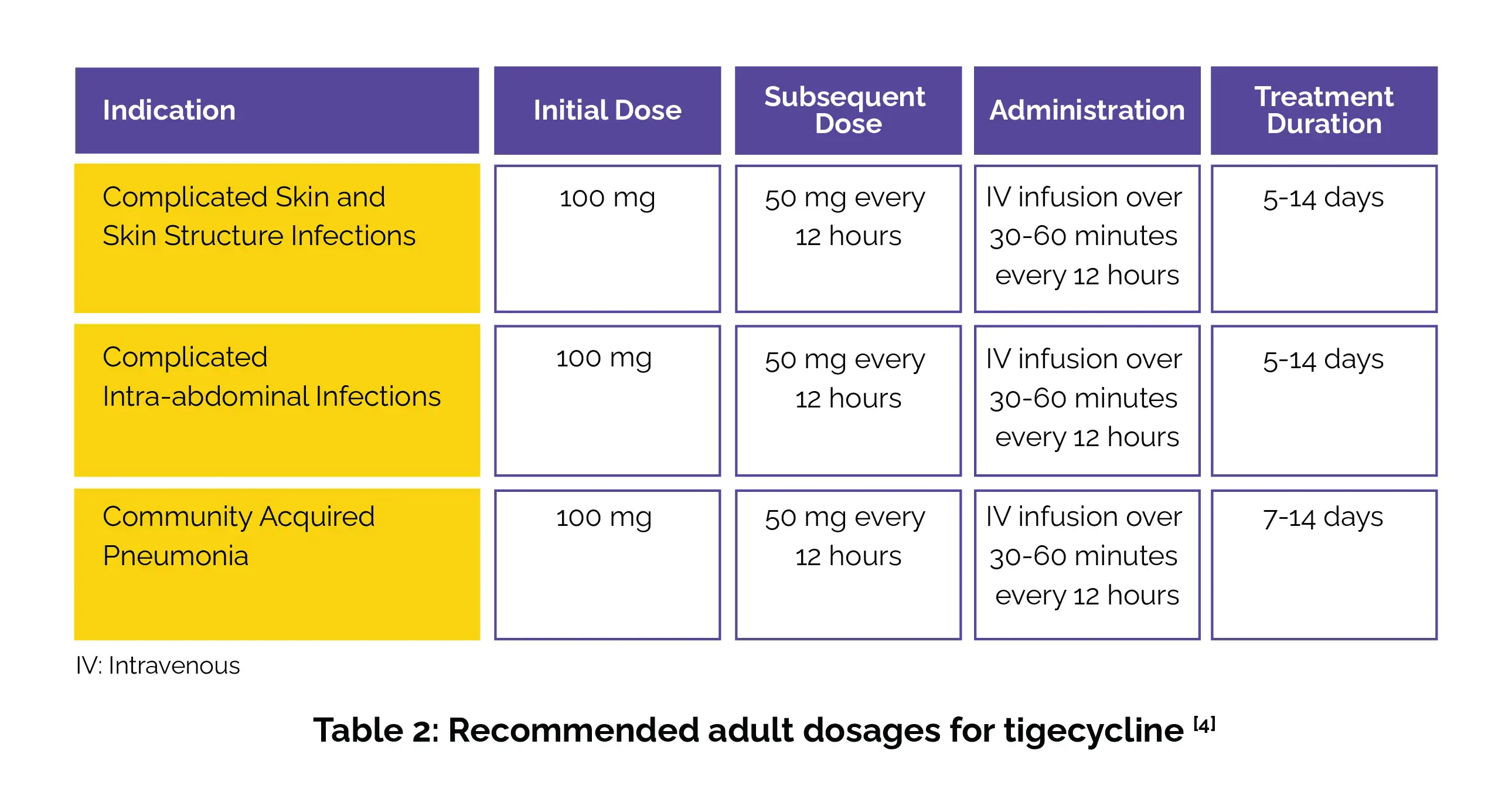
Tigecycline dosage varies depending on the condition being treated. Below is a summary of the recommended dosages (Table 2):
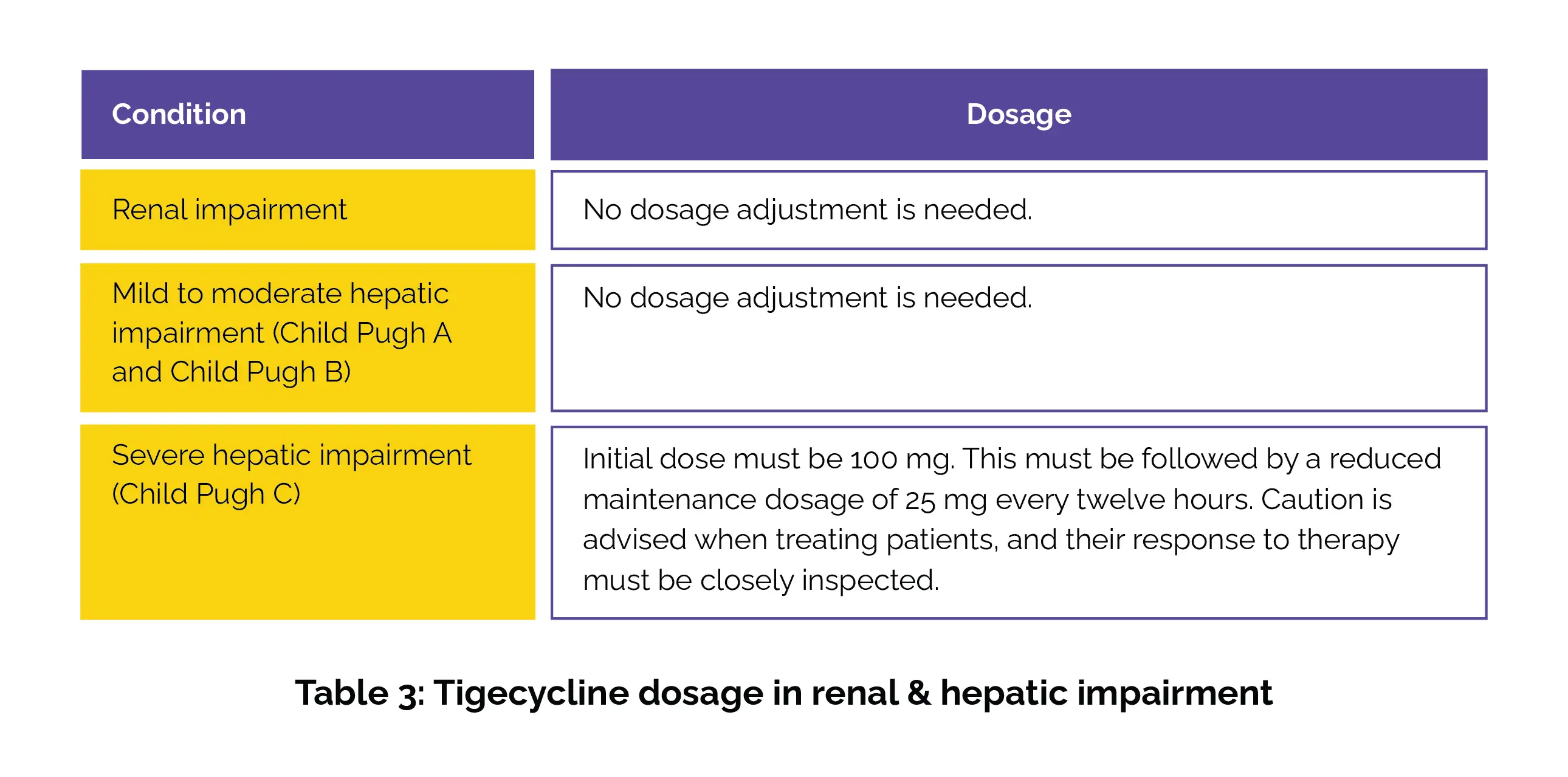
The duration of therapy should be based on the infection's severity and location, along with the patient's bacteriological and clinical response. Table 3 outlines tigecycline's dosage for those with renal and hepatic impairment.
Additional note: Tigecycline should not be used in pediatric patients unless no alternative antibacterial options are available. [4,5]
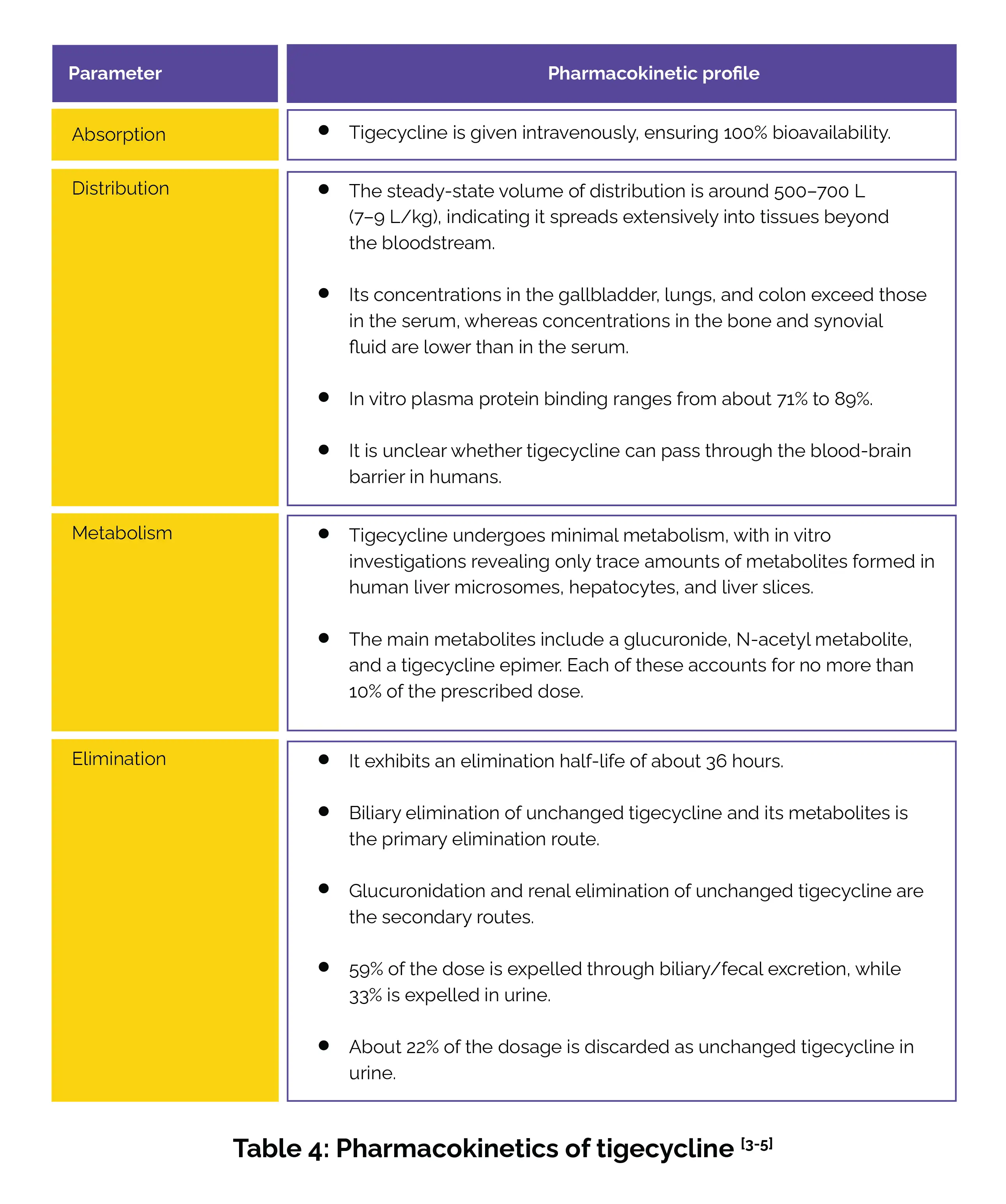
Table 4 presents a detailed overview of tigecycline’s pharmacokinetic profile.
Tigecycline injection is contraindicated for treatment of:
(a) Diabetic foot infections
(b) Hospital-acquired pneumonia or ventilator-associated pneumonia [4]
Note: In 2010 and 2013, the US FDA issued a boxed warning regarding an increased risk of death associated with tigecycline, particularly in patients with ventilator-associated pneumonia. [6]
Tigecycline is contraindicated in patients with a history of hypersensitivity to tetracycline-class antibiotics, as they may also be allergic to tigecycline. Precautions should be taken, and a thorough assessment for any history of allergic reactions to tetracyclines should be conducted before use. [5]
(A) Warfarin: If tigecycline is administered alongside warfarin, prothrombin time or another appropriate anticoagulation test should be monitored.
(B) Oral Contraceptives: Concomitant usage of antibacterial agents with oral contraceptives can render oral contraceptives less efficient. [4]
The most commonly reported side effects are:
[1] Tigecycline for Complicated Skin and Soft-Tissue Infections
In a study by Montravers P et al., tigecycline, either as monotherapy or in combination, attained favorable clinical response rates in complicated skin and soft-tissue infections patients with severe illness. Researchers analyzed data from 254 patients treated with tigecycline for infections across 5 European observational studies. Patients had severe disease (mean Acute Physiology and Chronic Health Evaluation [APACHE] II: 15.0, Sequential Organ Failure Assessment [SOFA]: 5.8), with 34.4% in intensive care units and 54.5% acquiring infections in hospitals. The most frequently encountered infection sites were limbs (62.4%), and necrotizing infections occurred in 43.8%.
Staphylococcus aureus, Escherichia coli, and Enterococcus faecium were frequent pathogens, with 30.5% of cases involving resistant strains. Tigecycline was used as monotherapy (71.8%) or combination therapy (28.2%) for an average of 12 days. Clinical response rates were 79.6% overall, 86.7% for monotherapy, 75.0% for nosocomial infections, and 75.3% for APACHE II scores >15. These results portray tigecycline's effectiveness in tackling severe complicated skin and soft-tissue infections in real-world settings. [7]
[2] Tigecycline vs. Meropenem for Postoperative Complicated Intra-abdominal Infections
A study involving 56 patients with postoperative complicated intra-abdominal infections in abdominal tumor cases compared the efficacy and safety of tigecycline and meropenem. Patients were divided into 2 groups: 30 received meropenem, and 26 received tigecycline. The clinical success rates were 83.33% and 76.67% for meropenem, compared to 76.92% and 88.46% for tigecycline at the end-of-therapy and discharge visits, respectively. The study concluded that tigecycline is as effective and safe as meropenem for managing postoperative complicated intra-abdominal infections in patients with abdominal tumors, demonstrating non-inferiority to meropenem. [8]
[3] High-Dose Tigecycline (HDT) for Severe Infections
In a systematic review and meta-analysis, which included 10 studies with 593 patients, the use of HDT led to better outcomes when compared to controls for the treatment of severe infections. HDT was linked with lower all-cause mortality (Odds ratio [OR] 0.44), higher clinical cure rates (OR 3.43), and better microbiological eradication (OR 2.25), without increasing the rates of side effects. Subgroup analysis showed that HDT was particularly useful in treating nosocomial acquired pneumonia (OR 0.39), bloodstream infections (OR 0.19), and mixed infections (OR 2.04), while there were no significant differences in complicated intra-abdominal infections (OR 2.04). [9]
[4] Tigecycline versus Levofloxacin for Community-Acquired Pneumonia
In a randomized phase 3 study, tigecycline was compared to levofloxacin in 418 hospitalized community-acquired pneumonia patients. These patients received intravenous (IV) tigecycline or levofloxacin, with the option to switch to oral levofloxacin after at least six IV doses. Treatment lasted 7–14 days. Cure rates at test-of-cure were comparable between groups. In the clinically evaluable population, cure rates were 90.6% for tigecycline and 87.2% for levofloxacin. In the clinical modified intent-to-treat (c-mITT) population, the rates were 78% and 77.8%, respectively.
Adverse effects differed slightly: nausea and vomiting were more common in tigecycline- treated patients, while elevated alanine aminotransferase and aspartate aminotransferase levels were more frequent with levofloxacin. No vital differences were noted in hospital length of stay, duration of IV or oral antibiotics, hospital readmissions, or transitions to oral levofloxacin. Overall, tigecycline demonstrated safety, efficacy, and noninferiority to levofloxacin in managing community-acquired pneumonia. [10]
[5] Tigecycline for Multidrug-Resistant Urinary Tract Infections (UTIs)
A systematic review investigated 27 cases of UTIs aroused by multidrug-resistant bacteria. These included 13 complicated UTIs and 5 catheter-linked UTIs, primarily caused by Escherichia coli (n=6), Acinetobacter baumannii (n=9), and Klebsiella pneumonia (n=11). Tigecycline was administered as monotherapy in 19 cases and in combination with other antibiotics in 8 cases, with a median treatment duration of 13 days.
Favorable clinical or microbiological responses were witnessed in 88.9% of patients (24/27), though 4 experienced symptom recurrence within 3 months. Tigecycline showed potential as an effective option for treating UTIs caused by resistant organisms, particularly when alternative antibiotics are not viable. However, recurrence in some patients highlights the need for further investigation. [11]
Comments (0)