Categories
Change Password!
Reset Password!


In patients diagnosed with H. pylori infection, the use of Amoxicillin-Vonoprazan dual treatment was associated with safe and effective elimination of H. pylori.
In a systematic review and meta-analysis published in the Journal of Gastroenterology and Hepatology, Amoxicillin-Vonoprazan (VA) dual therapy exhibited good safety, adequate effectiveness, and also evaded the need for unnecessary usage of antibiotics in the first-line therapy of Helicobacter pylori (H. pylori) infection. Yaobin Ouyang et al. sought to assess safety and efficacy of Amoxicillin and Vancomycin combination for H. pylori elimination.
This meta-analysis included 3 trials and 668 H. pylori-infected individuals. The terms "Helicobacter pylori or H. pylori or Hp," "Amoxicillin or penicillin," and "Vonoprazan or TAK-438 or Takecab or (potassium AND competitive) or potassium-competitive" were used in the systematic search of the electronic databases like Cochrane, Embase, and Pubmed. The primary and secondary outcomes were assessment of effectiveness and safety of VA dual treatment.
According to the per-protocol (PP) and intention to treat (ITT) assessment, the crude elimination rate of VA dual treatment is depicted in Table 1:
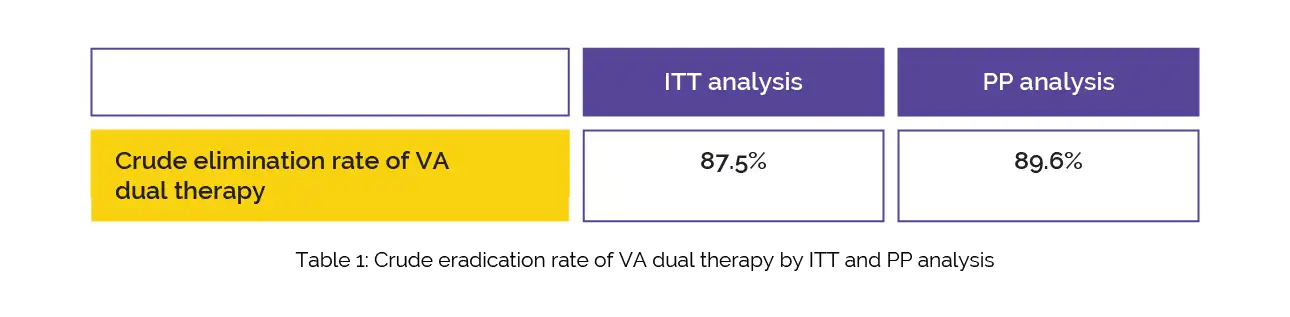
According to ITT (Risk Ratio [RR] = 0.99) and PP (RR = 0.99) analyses, there were no discernible differences between the VA dual therapy and Vonoprazan-Amoxicillin-Clarithromycin (VAC) triple therapy. Although the side effect rate of VA dual therapy was 19.1%, lower than that of VAC triple therapy, there was no statistically significant difference (RR = 0.75). Hence, VA dual therapy is promising for the management of H. pylori-infected people.
Journal of Gastroenterology and Hepatology
Amoxicillin-Vonoprazan dual therapy for Helicobacter pylori eradication: A systematic review and meta-analysis
Yaobin Ouyang et al.
Comments (0)