Categories
Change Password!
Reset Password!


The mucoprotective agent CKD-495 can be regarded as the preferred treatment for the symptomatic alleviation and healing of both acute and chronic erosive gastritis.
In a double-blind, randomized phase III trial, it was observed that CKD-495 at a dosage of 75 mg demonstrated a better endoscopic improvement rate for erosions when compared to Artemisiae argyi folium (AAF) at a dosage of 60 mg in patients with acute and chronic gastritis. The study aimed to examine the effectiveness and safety of CKD-495 in comparison to AAF for gastritis. In this multicenter, parallel-group trial, 242 patients with endoscopically confirmed gastric mucosal erosions underwent treatment with either CKD-495 at a dosage of 75 mg (n = 122) or AAF at 60 mg (n = 120), each administered three times daily along with a placebo (for double-blind conditions) over two weeks.
The key effectiveness endpoint was the rate of improvement in erosions. Secondary outcomes encompassed cure rates for erosions and improvement rates for oedema, redness, haemorrhage, and gastrointestinal symptoms. An assessment of adverse events related to the drug was also conducted.
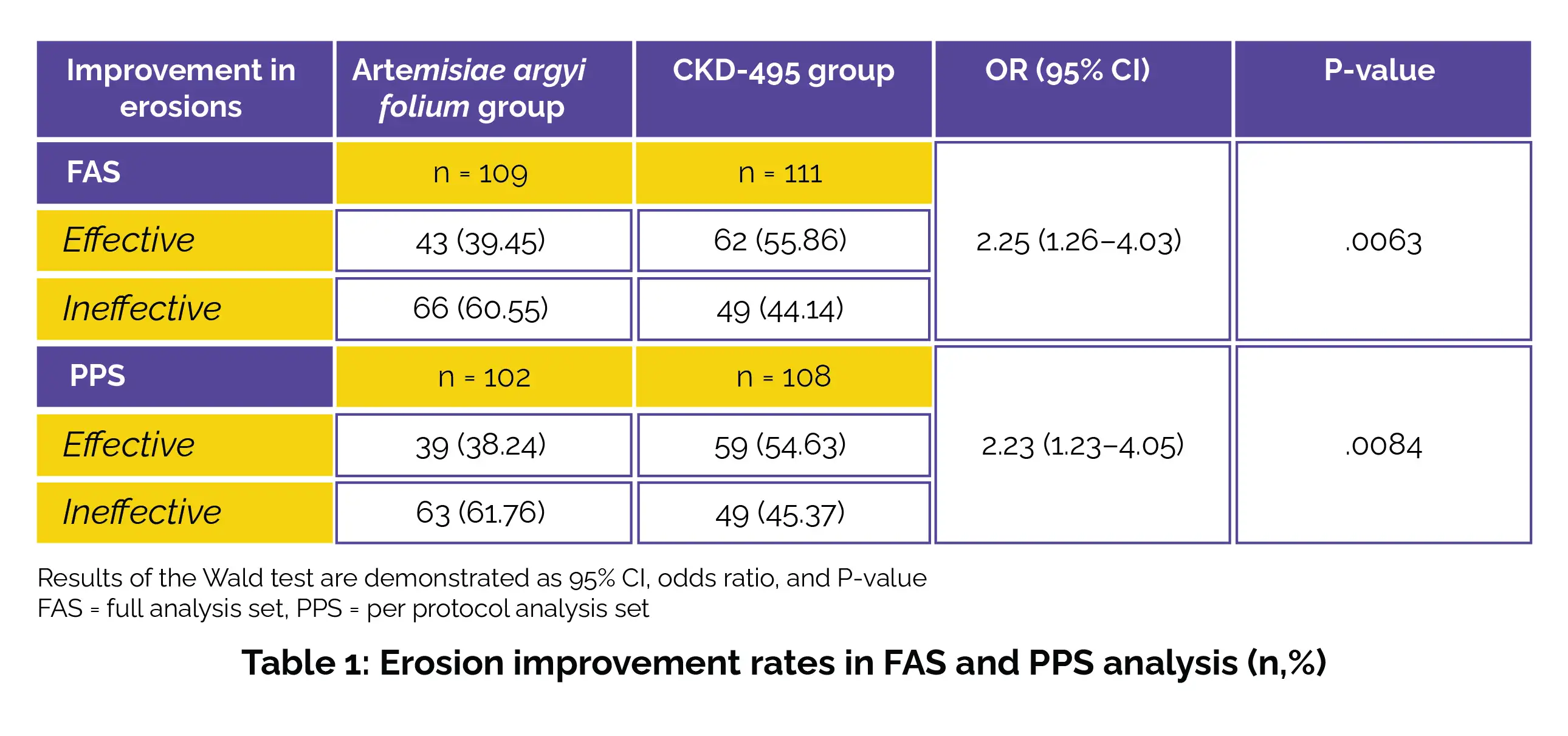
The analysis cohort was stratified into three groups: (i) Per-protocol analysis set (PPS), (ii) Full analysis set (FAS), and (iii) Safety set. The rates of endoscopic improvement in erosions were assessed using the Wald test, with the study site included as a covariate.
The outcomes were expressed in terms of a 95% confidence interval (95% CI), odds ratio (OR), and P value. Within the analysis of the FAS, individuals in the CKD-495 group displayed a superior rate of amelioration in erosions compared to those in the AAF group. Similar rates of erosion improvement were also noted in the PPS (Table 1).
The analysis also involved a comparison of erosion improvement rates among patients in the FAS with either acute or chronic gastritis. Those in the CKD-495 group exhibited a more pronounced improvement in erosion when compared to the AAF group (Table 2).

As found, subjects in the CKD-495 cohort exhibited better improvement rates compared to those in the AAF group in terms of oedema (OR, 2.54; 95% CI, 0.37–17.38), erosion cure rate (OR, 1.76; 95% CI, 0.98–3.17), haemorrhage (OR, 1.72; 95% CI, 0.57–5.17), redness (OR, 1.40; 95% CI, 0.50–3.86), and self-reported gastrointestinal symptoms (OR, 1.05; 95% CI, 0.58–1.90). Comparable results were noted in the PPS.
No severe adverse events or serious adverse drug reactions were documented in either of the treatment groups throughout the study duration. The occurrence of adverse events in the CKD-495 and AAF groups was 3.28% (4 out of 122) and 4.17% (5 out of 120), respectively. All reported adverse drug reactions were of mild intensity, and no substantial distinctions were observed between the two treatment groups. Therefore, CKD-495 (75 mg) outperformed AAF (60 mg) in improving endoscopic erosion rates in patients suffering from acute or chronic gastritis.
Medicine
Efficacy and safety of CKD-495 in acute and chronic gastritis: A Phase III superiority clinical trial
Seo et al.
Comments (0)