Categories
Change Password!
Reset Password!


Baricitinib + narrowband UV-B therapy remarkably improves response rates and reduces inflammatory markers in patients with nonsegmental vitiligo.
The findings of a retrospective controlled study have portrayed the efficacy, safety, and tolerability of combining Baricitinib with narrowband UV-B (NB-UVB) therapy for active nonsegmental vitiligo (NSV). The goal was to investigate the combination of Baricitinib (a selective Janus kinase 1/2 inhibitor) and NB-UVB for NSV management. Participants were divided into 2 groups:
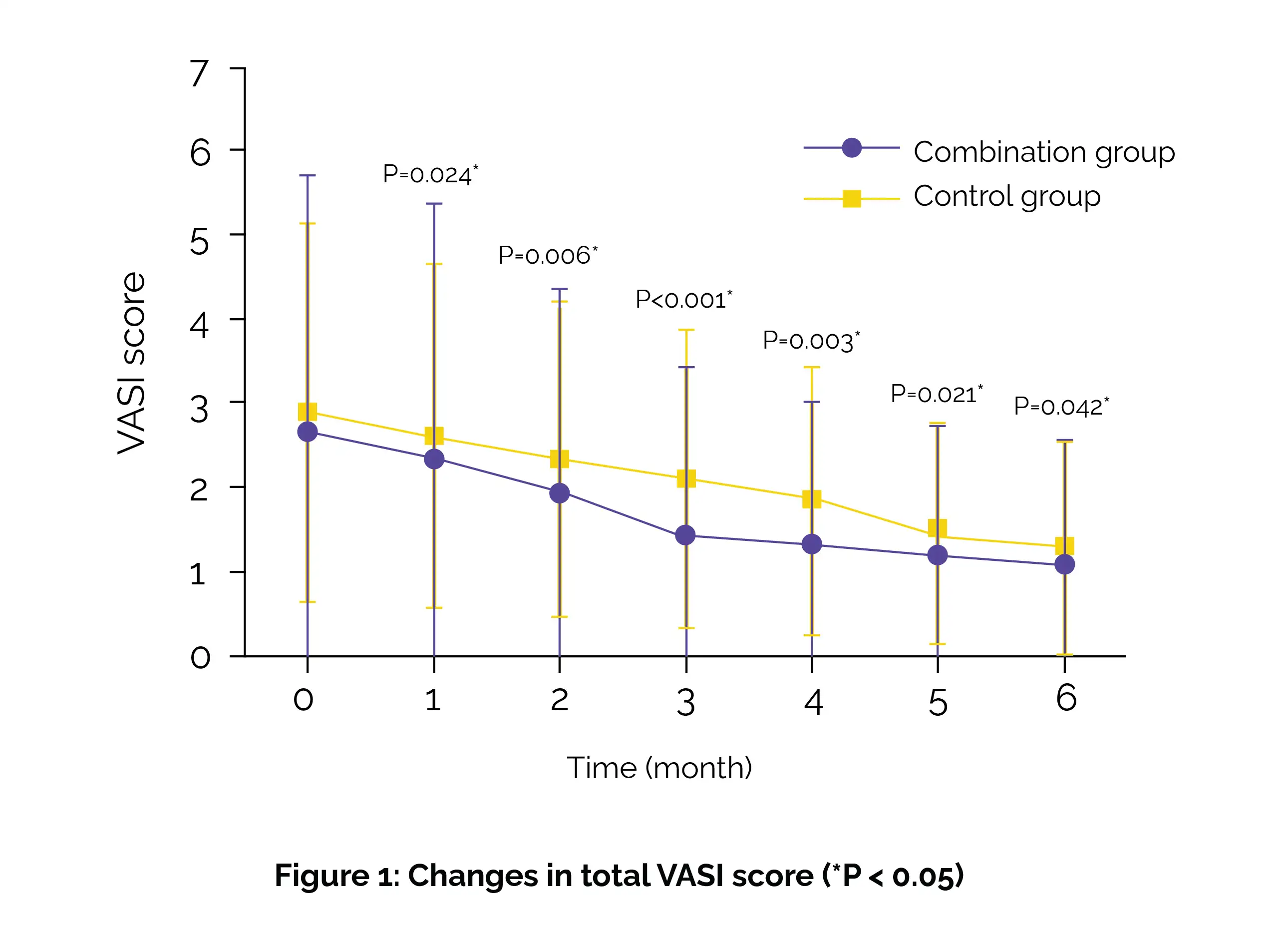
The efficacy was assessed over a 6-month treatment period, followed by a 6-month post-treatment follow-up to assess recurrence rates. From the first month, patients in the combination group showed a substantial decrease in vitiligo area scoring index (VASI) when compared to the control group (Figure 1).
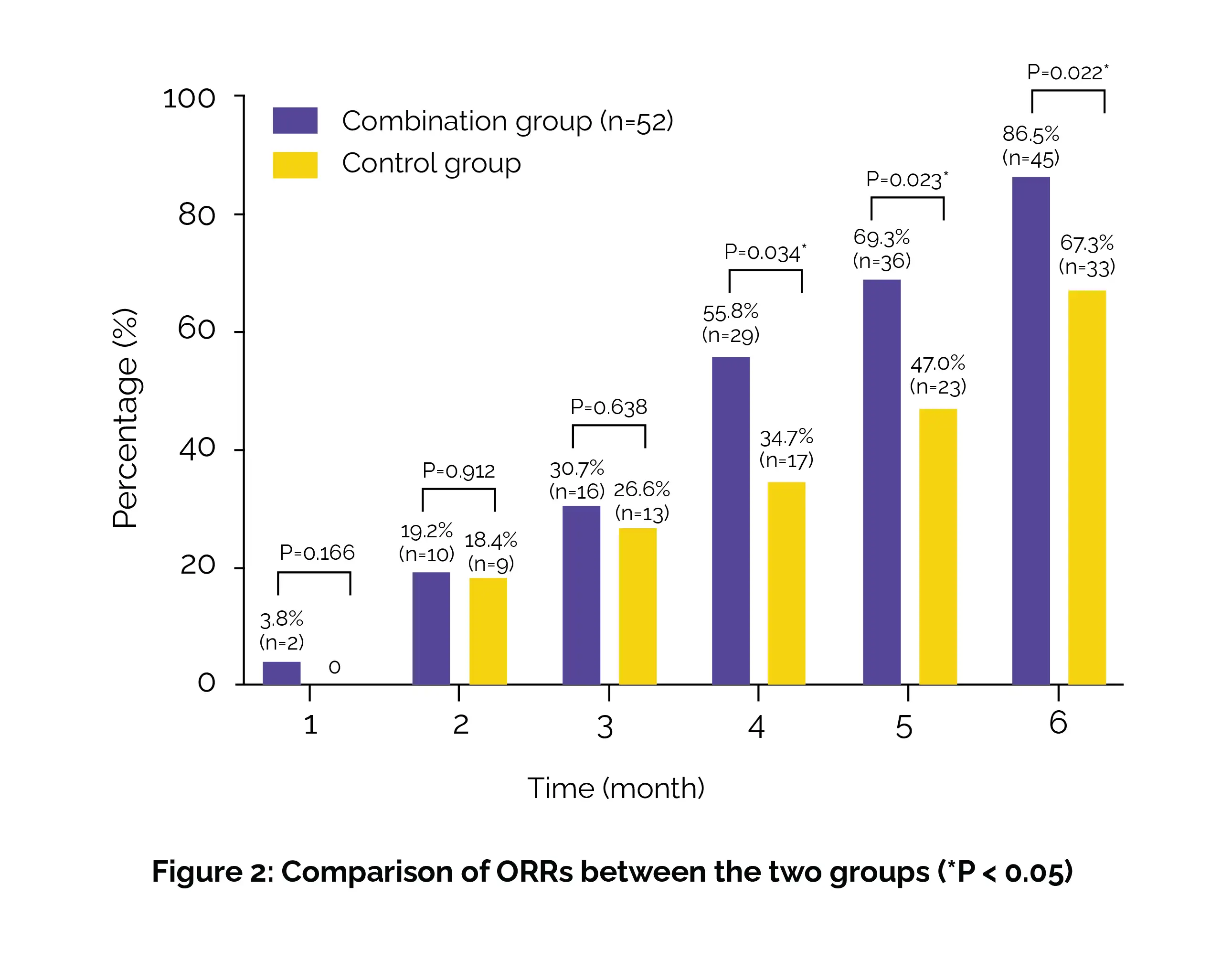
By the fourth month, the overall response rates (ORRs) were markedly higher in the combination group, reaching 86.5% by the sixth month compared to 67.3% in the control group (Figure 2).
In the combination group, the serum levels of interferon (IFN)-γ and C-X-C motif chemokine ligand 10 (CXCL10) markedly dropped from baseline values of 38.52 ± 5.98 pg/mL and 976.67 ± 150.57 pg/mL to 26.46 ± 5.93 pg/mL and 704.14 ± 103.38 pg/mL, respectively, at the 6-month mark. Recurrence rates within 6 months after treatment cessation demonstrated no vital differences between the groups.
Adverse events were minimal and included mild skin reactions such as itchiness (5.8%) and erythema (1.9%) in the combination group, with no serious adverse events reported. The findings position Baricitinib + NB-UVB as a promising and well-tolerated therapeutic option for NSV.
Clinical, Cosmetic and Investigational Dermatology
Combination Therapy with Baricitinib and Narrowband Ultraviolet B for Active Non-Segmental Vitiligo: A Retrospective Controlled Study
Bin Zhou et al.
Comments (0)