Categories
Change Password!
Reset Password!


Compared to darunavir-cobicistat, lopinavir-ritonavir was related to a shorter time to virological clearance and faster clinical improvement in COVID-19 patients.
A recent retrospective proof of concept study (DOLCI) published in "PLOS ONE" revealed that early treatment with lopinavir-ritonavir + standard of care was linked with shorter time to virological clearance and expedited clinical improvement than darunavir-cobicistat in patients hospitalized with SARS-CoV-2 pneumonia.
This multicenter observational study was performed to assess the safety and effectiveness of darunavir-cobicistat [800 mg darunavir/ 150 mg cobicistat 1 tablet orally once daily) in contrast to lopinavir-ritonavir (200 mg lopinavir/ 50 mg ritonavir 2 tablets orally twice daily) for COVID-19 management.
Overall, 400 adult participants who received darunavir-cobicistat (n = 100) or lopinavir-ritonavir (n= 300) for a minimum of 3 days as part of their COVID-19 intervention were involved and analyzed. From the electronic medical records of participants, collection of data was done. The composite endpoint of time to clinical improvement and/or SARS-CoV-2 clearance was the key endpoint ascertained.
As found, 92.5% of patients were male with a mean time from onset of symptoms to initiation of the therapy of 7.57 days. In contrast to individuals who were given darunavir-cobicistat, individuals who were given lopinavir-ritonavir exhibited a shorter time to virological clearance and/or clinical improvement (composite primary outcome). Lopinavir-ritonavir treated people exhibited a slower time to virological clearance and hastened time to clinical improvement than darunavir-cobicistat, as shown in Table 1:
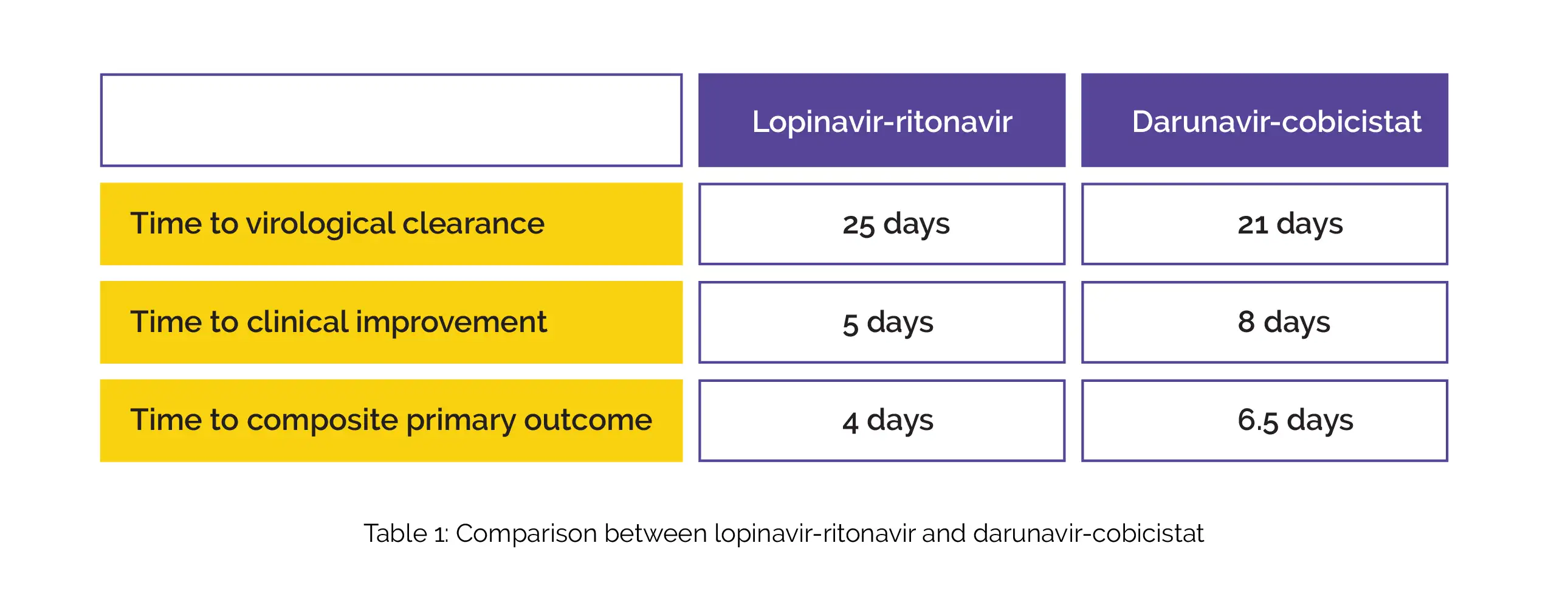
The safety profile of both the protease inhibitors was comparable since no profound inter-group differences in the occurrence of side effects were witnessed. Hence, lopinavir-ritonavir therapy is effective and safe to shorten time to virological clearance and accelerate clinical improvement in coronavirus-infected people.
PLOS ONE
Darunavir-cobicistat versus lopinavir-ritonavir in the treatment of COVID-19 infection (DOLCI): A multicenter observational study
Comments (0)