Categories
Change Password!
Reset Password!


Ebastine effectively relieves non-constipated irritable bowel syndrome symptoms.
In a recent research by Lisse Decraecker et al, Ebastine demonstrated superiority over placebo in offering relief for non-constipated irritable bowel syndrome (IBS)-affected people, with significantly more responders for abdominal pain intensity (API) and global relief of symptoms (GRS). This randomized, placebo-controlled, double-blind phase 2 study investigated the efficacy of Ebastine (histamine 1 receptor antagonist) as a potential treatment for non-constipated IBS.
The study, which encompassed participants meeting the Rome III criteria for non-constipated IBS, randomly allocated individuals to get either 20 mg of Ebastine or a placebo over a 12-week period. Throughout the study duration, the volunteers were examined for their GRS and API. A participant was deemed a weekly responder for GRS if they reported total or noticeable relief, and a responder for API if their weekly average pain score decreased by at least 30% compared to baseline.
The key outcome was to determine the percentage of participants who were weekly responders for at least six out of the twelve treatment weeks for both API and GRS (referred to as 'GRS+API', a composite outcome), as well as for API and GRS individually. Results from the study, which involved 202 participants (with an average age of 32 years and 68% female), demonstrated promising findings.
Those receiving Ebastine displayed a considerably higher proportion of responders for the composite endpoint of GRS + API compared to the placebo group. Additionally, while not statistically significant, Ebastine also demonstrated better proportions of responders for API and GRS separately, as shown in Table 1:
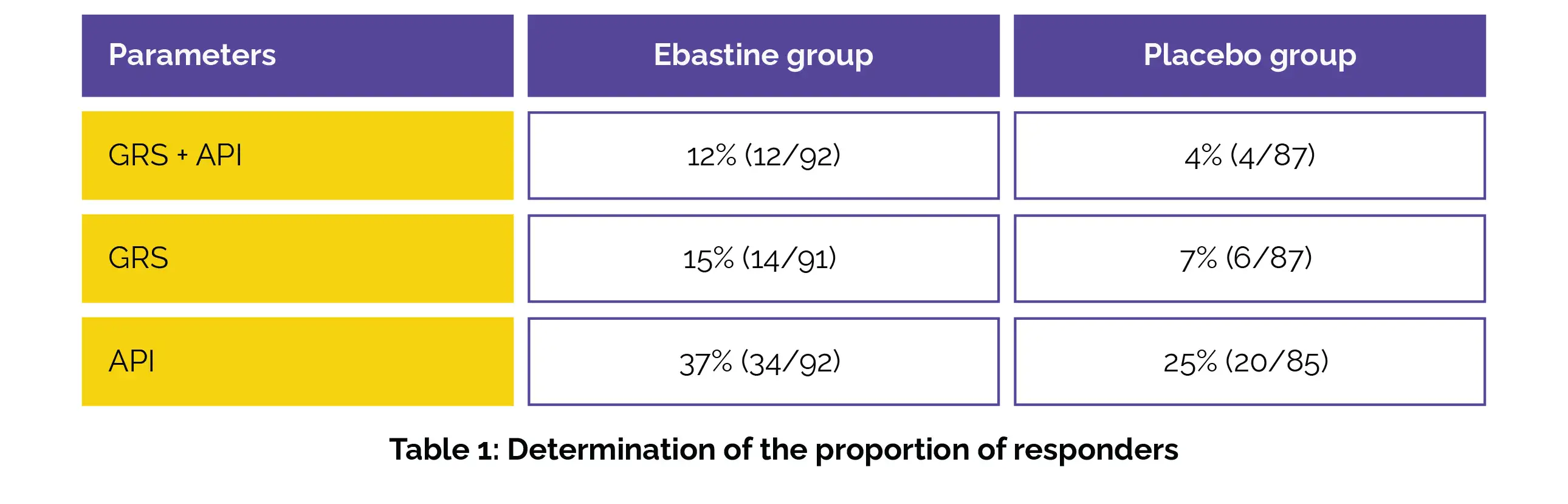
These findings suggest that Ebastine holds potential as a novel treatment option for patients suffering from non-constipated IBS. Further evaluation and research into Ebastine's effectiveness in alleviating IBS symptoms are warranted based on these encouraging results.
Gut
Treatment of non-constipated irritable bowel syndrome with the histamine 1 receptor antagonist ebastine: a randomised, double-blind, placebo-controlled trial
Lisse Decraecker et al.
Comments (0)