Categories
Change Password!
Reset Password!


Casirivimab plus Imdevimab used monthly can help prevent COVID-19.
A recent phase-1 study published in the International Journal of Infectious Diseases revealed that the monthly subcutaneous (SC) administration of Casirivimab along with Imdevimab (CAS + IMD) was found to be well-tolerated with low immunogenicity and also had a significantly lower risk of COVID-19 occurrence in uninfected adults. Flonza Isa and colleagues assessed the overall effectiveness of CAS + IMD used as frequent monthly SC doses in adult volunteers.
All in all, 969 individuals were randomized (3:1) to receive CAS+IMD 1200 mg or placebo every month for about 6 doses. Primary and secondary endpoints estimated the safety, pharmacokinetics, and immunogenicity. Exploratory efficacy was evaluated by the incidence of COVID-19 or seroconversion from negative to positive for SARS-COV-2 anti-nucleocapsid antibodies indicative of a SARS-CoV-2 infection.
The combination therapy has a relative risk reduction (RRR) of 92.4% and 100% in suspected and confirmed COVID-19, refer to Table 1 below:
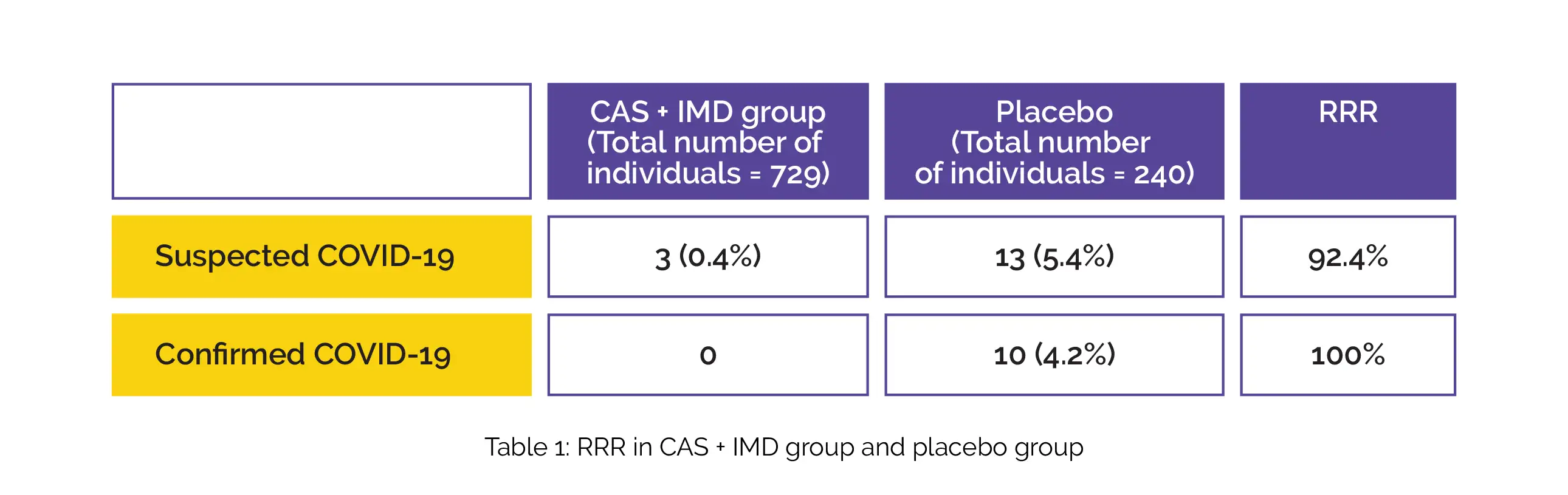
Due to lesser anti-drug antibodies developed, the combination group had low immunogenicity. There were no grade ≥3 injection-site reactions. However, more grade 1–2 injection-site reactions were reported in the combination group than placebo. No mortalities were reported.
International Journal of Infectious Diseases
Repeat subcutaneous administration of casirivimab and imdevimab in adults is well-tolerated and prevents the occurrence of COVID-19
Flonza Isa et al.
Comments (0)