Categories
Change Password!
Reset Password!


VX-548 effectively diminishes post-surgery pain, especially when administered at higher doses.
In an astonishing medical breakthrough, researchers have unveiled the stunning potential of a cutting-edge drug, VX-548, which has demonstrated a remarkable ability to alleviate acute pain in two groundbreaking clinical trials. The secret behind this game-changing discovery lies in the targeted inhibition of the NaV1.8 voltage-gated sodium channel, a key player in transmitting pain signals.
After conducting extensive in vitro tests to confirm VX-548's selectivity in inhibiting NaV1.8, Jim Jones et al. embarked on two ambitious phase 2 trials as below:
Abdominoplasty Trial: This trial included 303 participants who had undergone abdominoplasty surgery and were subjected to one of four treatment cohorts:
High-dose VX-548: Every 12 hours, participants received a loading dose of VX-548 100 mg orally and then, a maintenance dose of 50 mg
Middle-dose VX-548: Participants received a loading dose of VX-548 60 mg and then, a maintenance dose of 30 mg every 12 hours
Hydrocodone bitartrate plus Acetaminophen: Participants received Hydrocodone bitartrate 5 mg plus Acetaminophen 325 mg every 6 hours
Placebo: Participants received a placebo at 6-hour intervals
Bunionectomy Trial: This trial involved 274 participants who had undergone bunionectomy surgery and were subjected to one of five treatment cohorts:
Participants received a high dose of VX-548
Participants received a middle dose of VX-548
Low-dose VX-548: Participants received a loading dose of VX-548 20 mg orally, and then a maintenance dose of 10 mg every 12 hours
Hydrocodone bitartrate plus Acetaminophen: Participants received Hydrocodone bitartrate 5 mg plus Acetaminophen 325 mg every 6 hours
Placebo: Participants received a placebo at 6-hour intervals
The primary endpoint focused on the time-weighted sum of the pain-intensity difference (SPID) measured over a 48-hour period (SPID48), which was calculated as per the scores from the Numeric Pain Rating Scale. Pain intensity was evaluated at 19 time points following the initial dose of VX-548 or placebo. The substantial reduction in the time-weighted SPID48 achieved with high-dose VX-548 compared to the placebo group after abdominoplasty and bunionectomy surgeries are displayed in the following Table 1:
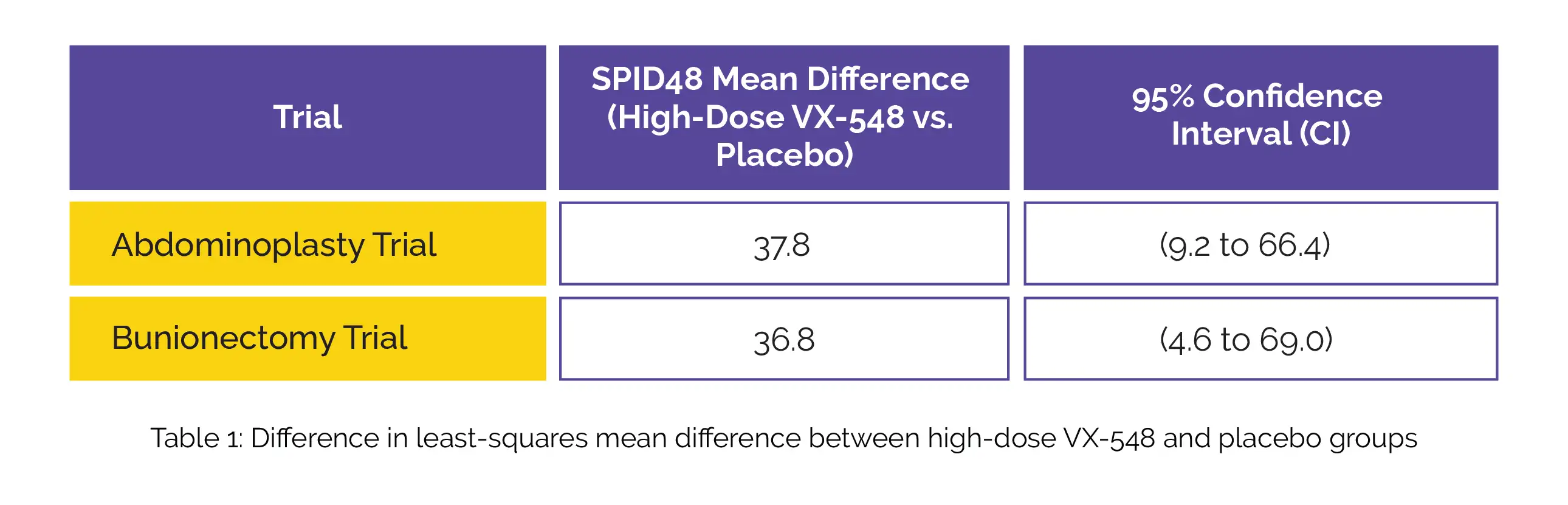
In both trials, the study participants who received lower doses of VX-548 displayed similar results as placebo. Mild adverse events (constipation and headache) were observed with the use of VX-548. These findings highlight the significant potential of high-dose VX-548 in reducing acute pain over 48 hours after the surgical procedures under consideration.
The New England Journal of Medicine
Selective Inhibition of NaV1.8 with VX-548 for Acute Pain
Jim Jones et al.
Comments (0)