Categories
Change Password!
Reset Password!


The combination of Nemolizumab and topical corticosteroids/calcineurin inhibitors markedly relieves inflammation and itch in moderate-to-severe atopic dermatitis.
In adults and adolescents suffering from atopic dermatitis, a recent study demonstrated that subcutaneous Nemolizumab, combined with topical corticosteroids (TCS) and topical calcineurin inhibitors (TCI), led to remarkable improvements in both inflammation and itch. Two international Phase 3 trials, ARCADIA 1 and ARCADIA 2, determined Nemolizumab's efficacy and safety in addressing atopic dermatitis. The aim of this report was to summarize the findings from the 16-week initial treatment phase of these studies.
The trials were 48-week, randomized, placebo-controlled, double-blind studies conducted across 22 countries, enrolling 1,728 volunteers aged 12 years and older. Those with moderate-to-severe atopic dermatitis and unsatisfactory response to topical steroids were randomized 2:1 to get Nemolizumab 30 mg subcutaneously (initial 60 mg loading dose) or a matching placebo every 4 weeks, along with background treatment of TCS with or without TCI (TCS-TCI background treatment). The key outcomes ascertained at week 16 included:
The key secondary endpoints encompassed a ≥4-point improvement in Peak Pruritus Numerical Rating Scale (PP-NRS) scores at weeks 1, 2, 4, and 16, PP-NRS scores below 2 at weeks 4 and 16, a ≥4-point improvement in Sleep Disturbance Numerical Rating Scale (SD-NRS) at week 16, and combined EASI-75 or IGA success with a ≥4-point PP-NRS improvement at week 16. In both trials, Nemolizumab substantially outperformed placebo in achieving the primary endpoints. The safety profile was comparable between Nemolizumab + TCS-TCI and placebo + TCS-TCI, as shown in Table 1:
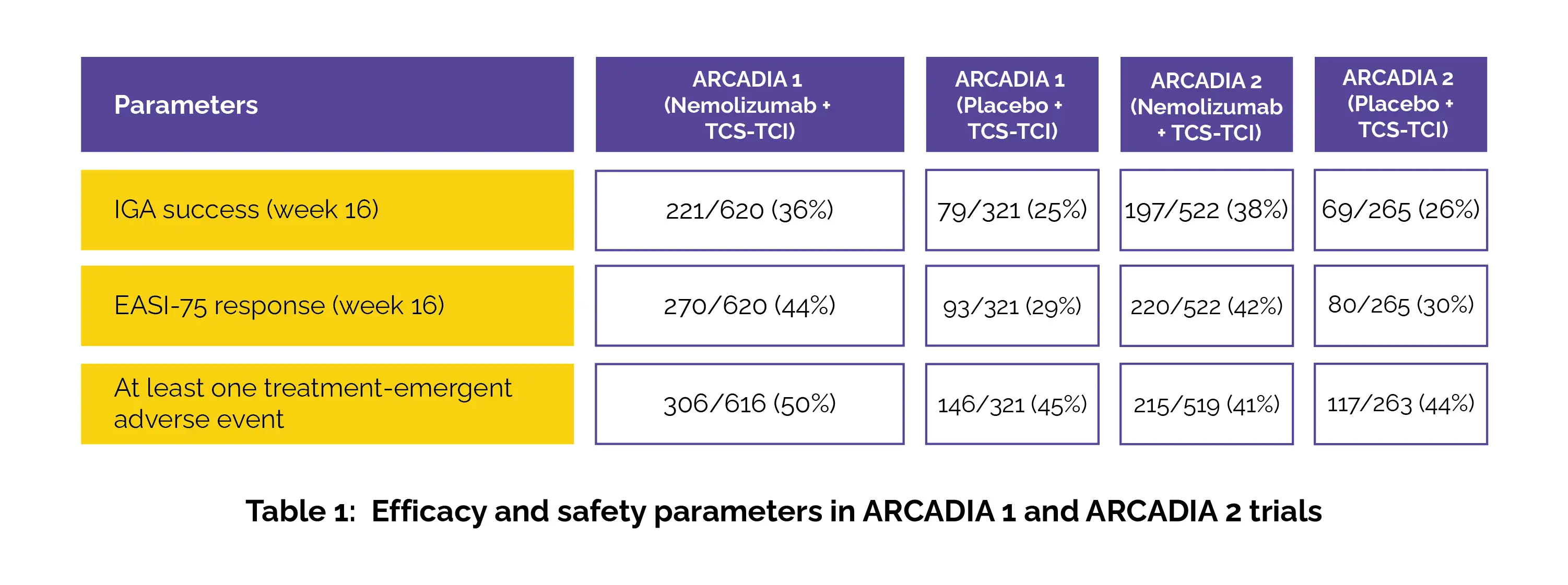
Secondary endpoints also depicted rapid improvements in pruritus, with drastic reductions in itch scores observed by week 1, and notable improvements in sleep by week 16. Serious treatment-related adverse events were rare, with no deaths reported. Nemolizumab (interleukin [IL]-31 receptor alpha antagonist) plus TCS-TCI exhibited significant clinical and statistical benefits in ameliorating inflammation and itch in atopic dermatitis, positioning it as a valuable addition to current therapeutic options if approved.
Lancet
Nemolizumab with concomitant topical therapy in adolescents and adults with moderate-to-severe atopic dermatitis (ARCADIA 1 and ARCADIA 2): results from two replicate, double-blind, randomised controlled phase 3 trials
Jonathan I Silverberg et al.
Comments (0)