Categories
Change Password!
Reset Password!


Effectiveness of Anaprazole is non-inferior to Rabeprazole in duodenal ulcer-affected people.
In Chinese patients with duodenal ulcers, Anaprazole exhibited non-inferior efficacy when compared to Rabeprazole, according to a randomized phase III study. This multicenter, double-blind, double-dummy, positive-drug parallel-controlled, non-inferiority trial compared the safety and effectiveness of the novel proton pump inhibitor (PPI) Anaprazole to Rabeprazole. The impact of CYP2C19 polymorphism and the presence of Helicobacter pylori (H. pylori) infection on Anaprazole was also assessed.
Overall, 448 duodenal ulcer-affected people were randomly allocated to get either 10 mg Rabeprazole + Anaprazole placebo (n = 223) or Rabeprazole placebo + 20 mg Anaprazole once a day (n=225) for four weeks. The four-week ulcer healing rate was the main effectiveness outcome and was determined by a blinded independent evaluation. The percentage of patients with reduced overall and specific duodenal ulcer symptoms after four weeks served as secondary outcomes.
In addition, an exploratory subgroup assessment of the key endpoint based on CYP2C19 polymorphism and H. pylori status was carried out. For safety, monitoring of adverse events was done. For the key endpoint, a non-inferiority analysis was carried out. Anaprazole was non-inferior to Rabeprazole, as evidenced by the 4-week healing rates (difference, -2.8%), and improvement in overall symptoms of duodenal ulcers, as shown in Table 1:
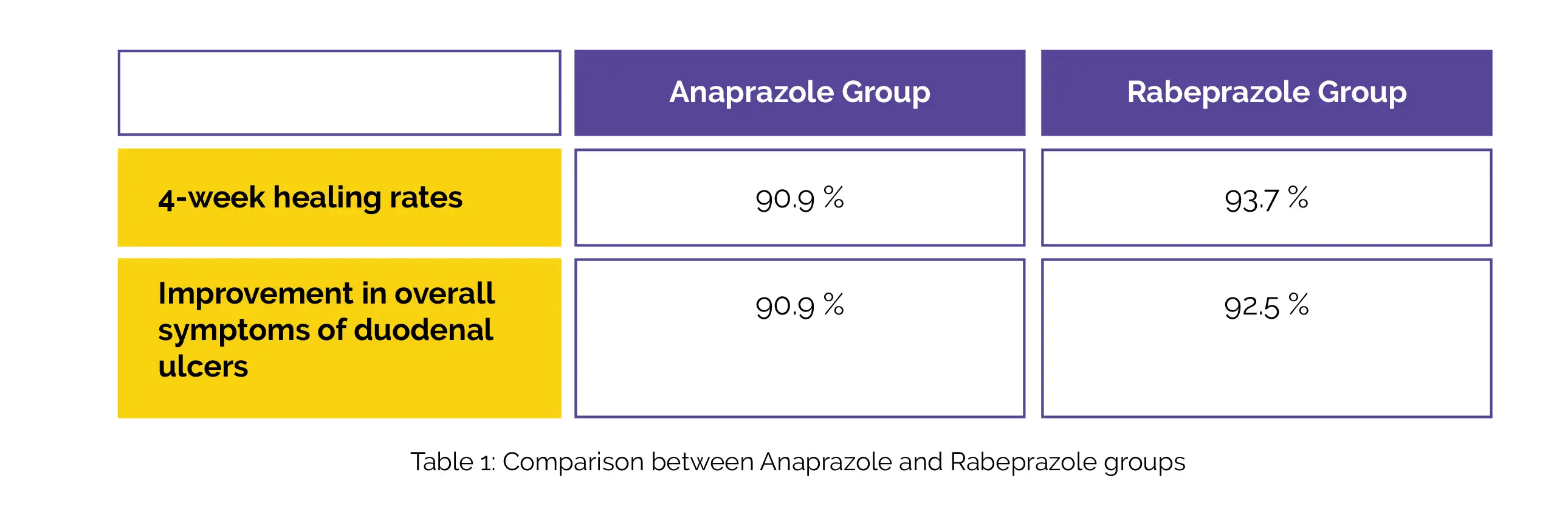
Individual symptom improvement rates were comparable between the groups. The healing rates did not considerably vary by CYP2C19 genotype or H. pylori status for either of the group. Anaprazole and Rabeprazole both had similar rates of treatment-emergent adverse events (72/220, 32.7% vs. 84/219, 38.4%). Hence, Anaprazole's effectiveness is comparable to Rabeprazole in treating duodenal ulcers.
The Chinese Medical Journal
Effect and safety of anaprazole in the treatment of duodenal ulcers: a randomized, rabeprazole-controlled, phase III non-inferiority study
Huiyun Zhu et al.
Comments (0)