Categories
Change Password!
Reset Password!


In patients with self-reported functional gastrointestinal disorders, Bacillus Subtilis improves particular symptoms associated with constipation and related gastrointestinal dysfunction.
In a phase 1/2A randomized controlled trial, the intake of a proprietary probiotic strain, Bacillus Subtilis [B. Subtilis, BG01-4™], was well-tolerated and effectively alleviated symptoms associated with constipation and related gastrointestinal dysfunction. The researchers aimed to assess the efficacy of BG01-4™ in sachet form in alleviating functional gastrointestinal disorders (FGID) symptoms.
A total of 67 volunteers underwent a 4-week intervention, with 34 receiving BG01-4™ and 33 receiving a placebo. The Gastrointestinal Symptom Rating Scale (GSRS) was used as the assessment tool, with data collected before the intervention, at 2 weeks, and 4 weeks following its completion. Following 4 weeks, the experimental group showed a 33% improvement in one of the three major endpoints—constipation—compared to the placebo group (15%).
Both other major endpoints, diarrhea and total GSRS, exhibited substantial betterment in both the placebo and experimental groups (22%/20% and 26%/32%, respectively). The pre-planned secondary endpoint, indigestion, showed improvement at 4 weeks (32%), but the difference in comparison with the placebo (21%) did not reach significance.
As found, dyspepsia (with a 10% improvement), indigestion (with an 11% improvement), and constipation (with an 18% improvement) clusters showed significant enhancements in the intervention group in comparison with the placebo, as shown in Figure 1:
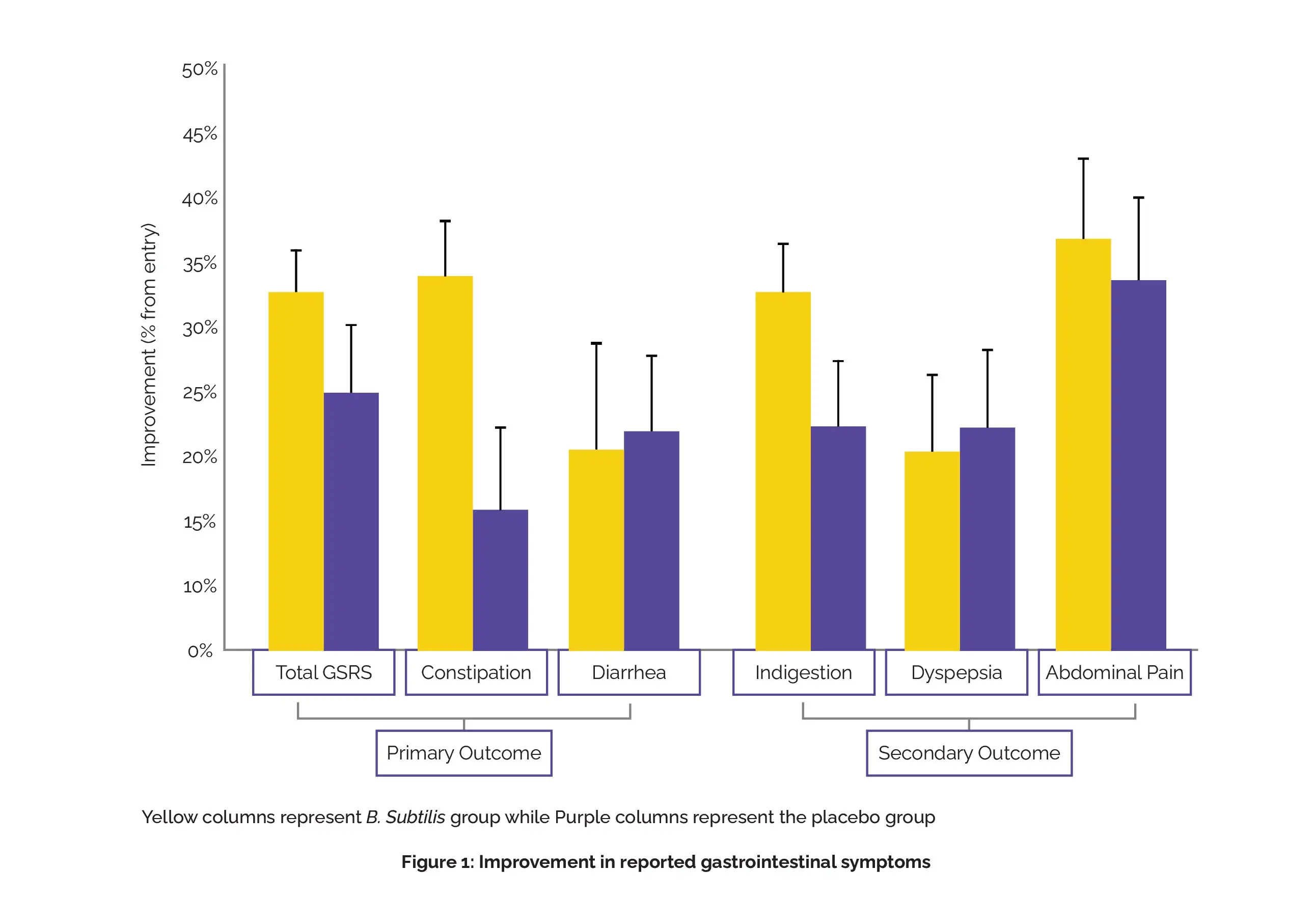
Hence, BG01-4TM remarkably improves self-reported symptoms of constipation, indigestion, and dyspepsia. Further, more long-term confirmatory studies are needed.
Nutrients
Bacillus Subtilis (BG01-4TM) Improves Self-Reported Symptoms for Constipation, Indigestion, and Dyspepsia: A Phase 1/2A Randomized Controlled Trial
Craig Patch et al.
Comments (0)