Categories
Change Password!
Reset Password!


Intravenous Ibuprofen, whether 400 mg or 800 mg, offer effective and safe analgesia when given pre-emptively during the perioperative phase of abdominal and orthopedic surgery.
A phase III randomized clinical trial showed that compared to placebo, intravenous Ibuprofen 400 mg and 800 mg substantially decreased pain scores at rest and with movement, both visual analog scale (VAS) and the area under the curve (AUC) of VAS on postoperative days 1 and 2, as well as Morphine consumption in patients undergoing abdominal and orthopedic surgery. The goal of Hong-Su Zhou et al. was to assess the analgesic effectiveness and safety of various intravenous Ibuprofen doses in the management of postoperative acute pain.
Following orthopedic or abdominal surgery, subjects who were using an intravenous patient-controlled analgesia device were arbitrarily assigned to intravenous 800 mg Ibuprofen, intravenous 400 mg Ibuprofen, or placebo. The first dose of study medications was administered intravenously 30 minutes prior to the operation, with subsequent dosages administered at six-hours intervals for 8 doses. During the trial period, the recording of demographic information, procedure information, cumulative Morphine intake, the VAS, the AUC of VAS, the patient satisfaction score (PSS), rates of treatment failure (RTF), adverse events, and serious adverse events was done.
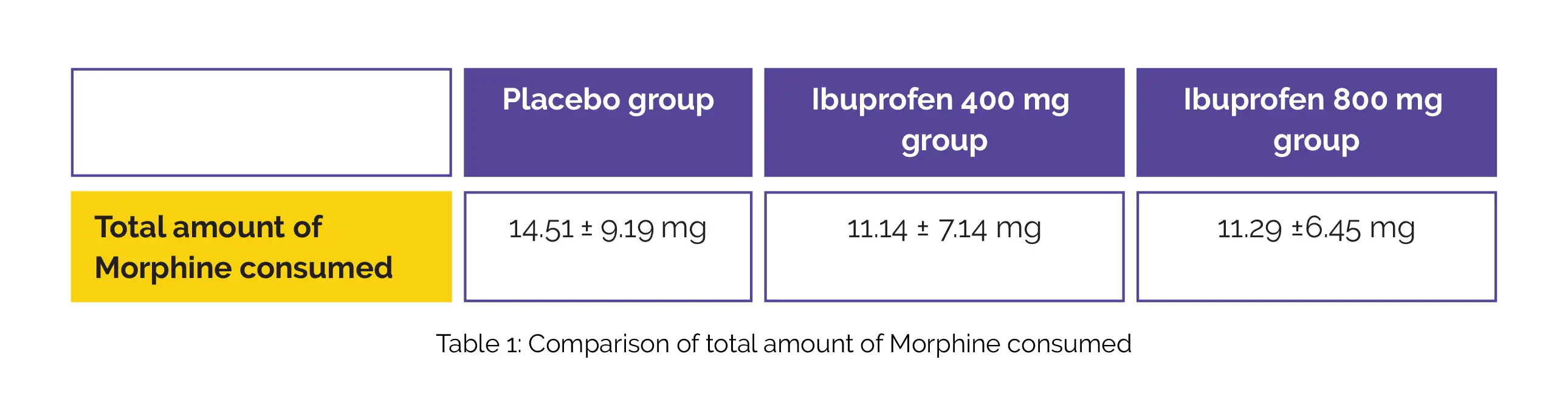
In this multicenter, placebo-controlled, double-blind study, the full analysis set included 345 individuals in total, and 326 of them were valid data set. Patients' demographic details, disease traits, and medical backgrounds did not substantially vary between groups. Compared with the placebo group, the total amount of Morphine consumed for 24 hours after surgery was reduced in Ibuprofen 400 mg and 800 mg groups. However, there was no noteworthy difference between the 400 mg and 800 mg Ibuprofen groups, as shown in Table 1:
The placebo group exhibited significantly greater VAS and AUCs of VAS when compared to the 400 mg and 800 mg Ibuprofen groups at rest and movement for 24 hours postoperatively. But, there was no vital difference between the Ibuprofen 400 mg and 800 mg groups. RTF was somewhat greater in the placebo group compared to the other two groups, but it was not clinically meaningful.
For pain relief, PSS was greater in Ibuprofen 400 mg and 800 mg groups than in the placebo group; however, there was no discernible difference between Ibuprofen 400 mg and 800 mg groups. The three groups did not vary in the frequency of RTF or adverse events. Hence, in patients scheduled to undergo abdominal and orthopedic surgery, intermittent intravenous use of Ibuprofen 400 mg or 800 mg within 24 hours of surgery dramatically reduced Morphine consumption and alleviated pain without elevating the occurrence of adverse events.
Pain Research and Management
Analgesic Efficacy of Intravenous Ibuprofen in the Treatment of Postoperative Acute Pain: A Phase III Multicenter Randomized Placebo-Controlled Double-Blind Clinical Trial
Hong-Su Zhou et al.
Comments (0)