Categories
Change Password!
Reset Password!


In patients with gastritis, Esomeprazole is non-inferior to Famotidine.
A randomized, double-blind, noninferiority, multicenter, phase 3 study demonstrated that the efficacy and safety of DW1903 (low-dose proton pump inhibitor [PPI]) were not inferior to those of DW1903R1 (histamine type 2 receptor antagonist) in individuals suffering from gastritis. Investigators aimed to assess the effectiveness and safety of utilizing a low-dose PPI in gastritis patients.
In total, 476 individuals diagnosed with endoscopic erosive gastritis were randomly allocated into two groups: one receiving a daily dose of Esomeprazole 10 mg (DW1903) and the other receiving a daily dose of Famotidine 20 mg (DW1903R1) over a span of 2 weeks. The full-analysis set encompassed 319 subjects (DW1903 group: n=159; DW1903R1 group: n=160), and the per-protocol set comprised 298 volunteers (DW1903 group: n=147; DW1903R1 group: n=151).
Following the treatment period, the major outcome, which focused on the rate of improvement in erosions, as well as secondary outcomes encompassing rates of cure for erosions and edema, betterment in hemorrhage and erythema, and relief in symptoms, were evaluated. The incidence of adverse events was also assessed. In terms of erosion improvement rate, DW1903 was not inferior to DW1903R1.
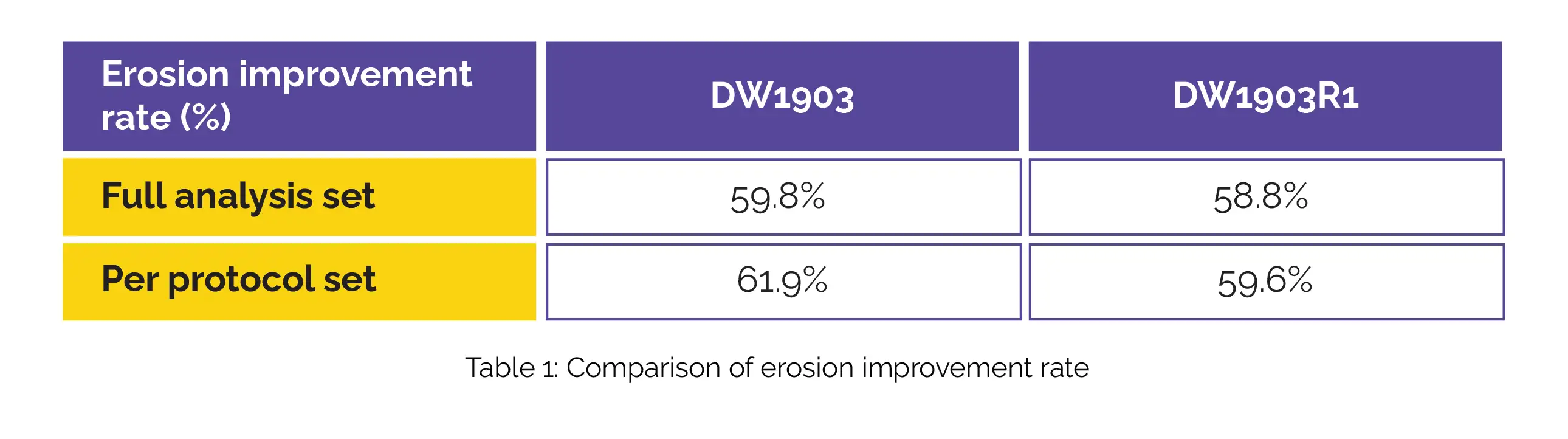
In the context of the full-analysis set, the DW1903 group demonstrated an erosion improvement rate of 59.8%, while the DW1903R1 group exhibited a rate of 58.8%. When considering the per-protocol analysis, the DW1903 group showcased a rate of 61.9% for erosion improvement, while the DW1903R1 group had a rate of 59.6%, as shown in Table 1:
Secondary endpoints did not exhibit substantial disparities between the two groups, with the exception of the hemorrhagic improvement rate, which displayed a higher tendency in the DW1903 group. There was no vital difference in the occurrences of adverse events. Therefore, using a low-dose PPI could represent a new and innovative approach to relieve gastritis.
Gut and Liver
Evaluation of the Efficacy and Safety of DW1903 in Patients with Gastritis: A Randomized, Double-Blind, Noninferiority, Multicenter, Phase 3 study
Jie-Hyun Kim et al.
Comments (0)