Categories
Change Password!
Reset Password!


In hospitalized COVID-19 patients, baricitinib + remdesivir and dexamethasone + remdesivir led to comparable mechanical ventilation-free survival by day 29.
A randomized, double-blind, double placebo-controlled trial (ACTT-4) illustrated that both baricitinib and dexamethasone (in combination with remdesivir) were associated with similar mechanical ventilation-free survival by day 29 in adults hospitalized with coronavirus infection and who needed supplemental oxygen. Researchers aimed to evaluate efficacy and safety of baricitinib + remdesivir versus dexamethasone + remdesivir to prevent progression to death or mechanical ventilation in COVID-19.
Out of 1047, 1010 hospitalized patients (aged ≥18 years, mean age 58·3 years) who need supplemental oxygen given by high-flow (>15 L/min), low-flow (≤15 L/min), or non-invasive mechanical ventilation modalities were recruited. Participants were randomly divided (1:1) into baricitinib + remdesivir + placebo group (n = 516, 51%) and dexamethasone + remdesivir + placebo group (n = 494, 49%).
The recruited volunteers were administered remdesivir (≤ten days) and either dexamethasone (or matching intravenous placebo) for maximum of ten days or baricitinib (or matching oral placebo) for maximum of fourteen days. In modified intention-to-treat population, the inter-group difference in mechanical ventilation-free survival was the major endpoint ascertained. Safety evaluation was carried out in the as-treated population, encompassing all the people who were administered 1 dose of the study drug.
By day 29, both the study groups had comparable mechanical ventilation-free survival (risk difference 0·6). Table 1 shows the percentage of people with at least 1 adverse event (risk difference 7·5%), at least one treatment-related adverse event (risk difference 6·0%), and severe or life-threatening grade 3 or 4 adverse events (risk difference 7·7%).
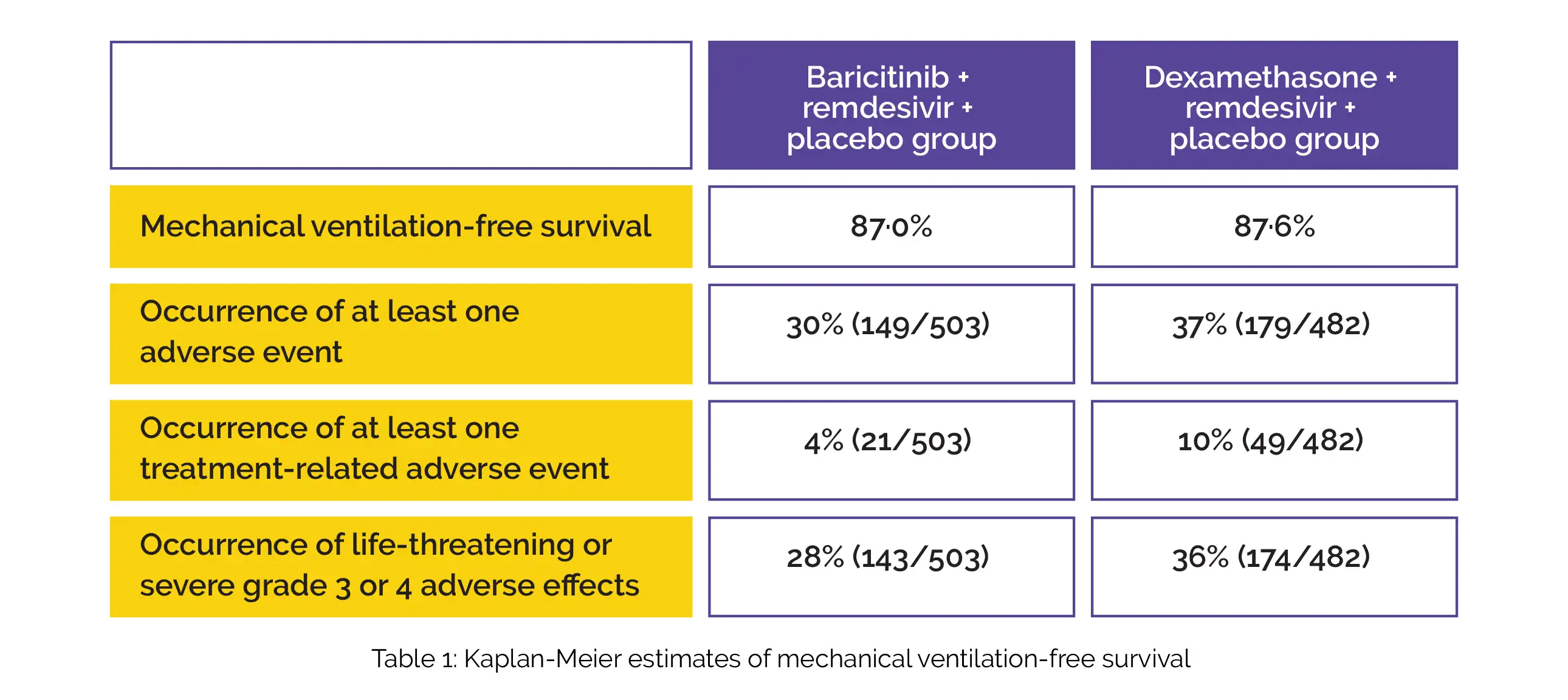
In contrast to the baricitinib + remdesivir + placebo group, the odds ratio for enhanced status in the dexamethasone + remdesivir + placebo group was found to be 1·01. To conclude, in SARS-CoV-2 infected people who require supplemental oxygen therapy, baricitinib in combination with remdesivir and dexamethasone in combination with remdesivir exhibited comparable efficacy in terms of mechanical ventilation-free survival. However, dexamethasone was related to more adverse events.
The Lancet
Baricitinib versus dexamethasone for adults hospitalized with COVID-19 (ACTT-4): a randomised, double-blind, double placebo-controlled trial
Cameron R Wolfe et al.
Comments (0)