Categories
Change Password!
Reset Password!


TNX-102 SL (sublingual formulation of Cyclobenzaprine) is well-tolerated and can effectively promote recovery from military-linked posttraumatic stress disorder.
As per the findings of a phase 3 randomized, placebo-controlled trial, monotherapy with 5.6 mg TNX-102 SL taken daily at bedtime can remarkably improve sleep disturbances in people suffering from posttraumatic stress disorder (PTSD), particularly those dealing with military-related trauma. This is a crucial finding, as sleep disturbances are a common and debilitating symptom of PTSD.
Megan E. Parmenter and other researchers examined the safety and efficiency of bedtime sublingual formulation of Cyclobenzaprine (TNX-102 SL) in military-linked PTSD at 44 U.S. outpatient psychiatric research clinics. Participants (aged between 18 and 75 years old) were randomly allocated in a 1:1 ratio to receive either 5.6 mg of TNX-102 SL (2 x TNX-102 SL 2.8 mg tablets) or placebo (2 x placebo SL tablets) for a duration of twelve weeks. Study visits occurred at screening, baseline, and at 2 (telephonic), 4, 8, and 12 weeks, or at the time of discontinuance if prior to week 12.
Follow-up visits were conducted one week after the completion of the study for those not entering the twelve-week open-label extension study. While the primary assessment investigating alteration from baseline in Clinician Administered PTSD Scale [CAPS-5] total severity between study groups at week 12 did not show profound differences, there were notable improvements observed as early as week 4, particularly in sleep-related symptoms, as shown in Figure 1:
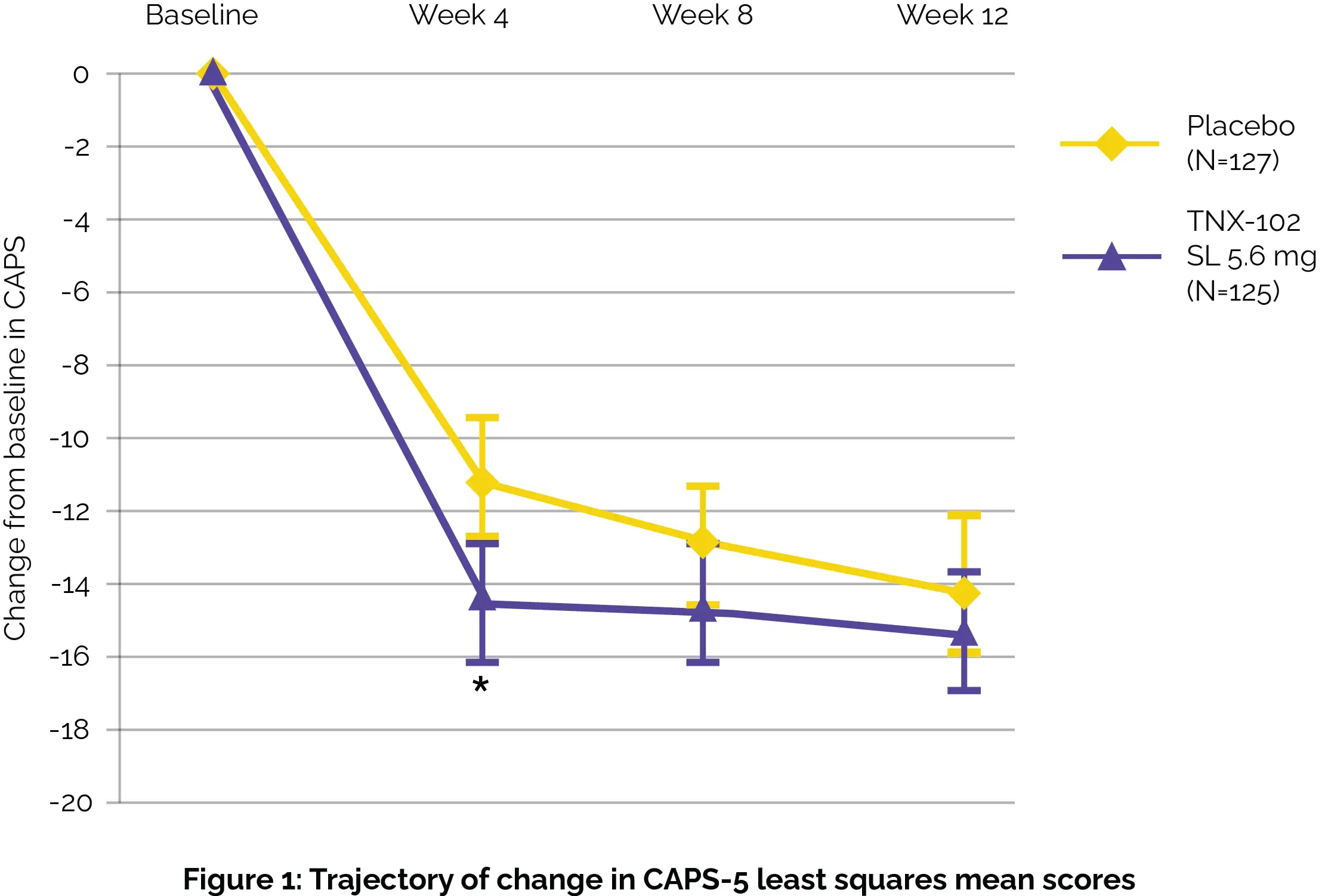
Secondary assessments further supported the potential benefits of TNX-102 SL use, showing improvements in both clinician and patient-reported outcomes (i.e. Clinician Global Impression of Improvement [CGI-I] at the 4th week and the Patient Global Impression of Change [PGIC] at the 12th week). No severe adverse events were documented. Importantly, TNX-102 SL was well-tolerated among participants, suggesting it could be a viable option for addressing PTSD symptoms.
The period since the occurrence of trauma acted as a factor distinguishing the therapeutic response according to the CAPS-5 assessment in the subgroup of individuals with ≤ 9 years since the initial event. Hence, targeting sleep disturbances with pharmacotherapy like TNX-102 SL appears to be a promising avenue for promoting recovery and improving overall quality of life.
Psychiatry Research
A phase 3, randomized, placebo-controlled, trial to evaluate the efficacy and safety of bedtime sublingual cyclobenzaprine (TNX-102 SL) in military-related posttraumatic stress disorder
Megan E. Parmenter et al.
Comments (0)