Categories
Change Password!
Reset Password!


Tofacitinib therapy is safe and helps in the reduction of the inflammatory response during coronavirus disease.
In hospitalized adults suffering from mild to moderate COVID-19 pneumonia, the combination of tofacitinib and standard of care (SOC) is safe and helps to effectively reduce the overwhelming inflammatory response during COVID-19 infections, according to the findings of an open-label randomized controlled study. Hema Murugesan et al. investigated the efficacy and safety of tofacitinib along with SOC for COVID-19 management.
Overall, 100 volunteers were randomized to either: (a) Study Group (N=50) : Received tofacitinib + SOC, and (b) Control Group (N=50): Received SOC only. People reporting positive reverse transcription polymerase chain reaction (RT-PCR) for SARS-COV-2 and radiological evidence of pneumonia were hospitalized for over seven days. Irrespective of discharge status, tofacitinib was given to the study group for 14 days and were followed up to 28 days.
A higher relative decrease was reported in the levels of pivotal markers of inflammation in the tofacitinib group when compared to the control group, as shown in Table 1:
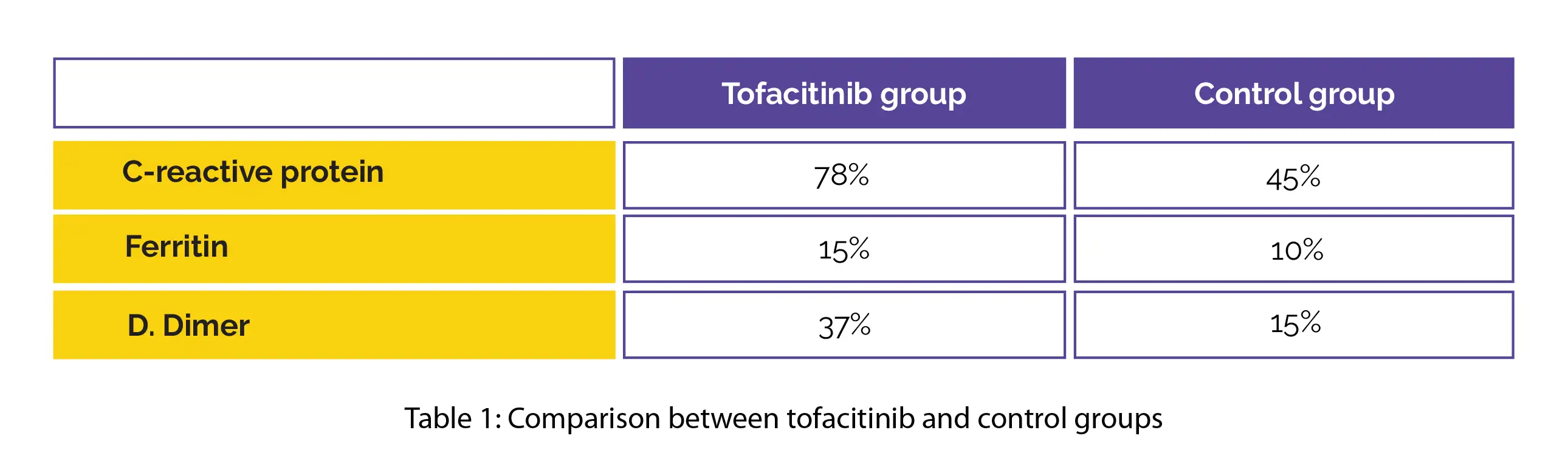
However, no differences in duration of hospital admission or oxygen need were noted. The Janus kinase inhibitor, tofacitinib (10 mg) has promising immunomodulatory potential, showed good tolerability, and was devoid of any severe adverse events. Thus, tofacitinib + SOC is effective and safe for management of hospitalized COVID-19 people.
The Journal of the Association of Physicians of India
An Evaluation of Efficacy and Safety of Tofacitinib, A JAK Inhibitor in the Management of Hospitalized Patients with Mild to Moderate COVID-19 - An Open-Label Randomized Controlled Study
Hema Murugesan et al.
Comments (0)