Categories
Change Password!
Reset Password!


When compared to Tenofovir disoproxil fumarate, Pradefovir demonstrated good effectiveness and an acceptable safety profile in patients with chronic hepatitis B.
Pradefovir and Tenofovir disoproxil fumarate (TDF) both demonstrated comparable reductions in hepatitis B virus (HBV) DNA levels and were safe and well-tolerated, as elucidated from a phase 2 study published in "Clinical Infectious Diseases". In this study, the effectiveness and safety of orally administered Pradefovir (30, 45, 60, or 75 mg) were compared against 300 mg of Tenofovir disoproxil fumarate in order to determine the best dose of Pradefovir for the next phase 3 investigation.
The study included therapy-naive and experienced (not on therapy for more than six months) people suffering from hepatitis. Overall, 240 people (48 per group) were randomized and studied. Approximately 80% of them tested positive for the hepatitis B e antigen (HBeAg), and 10% developed liver cirrhosis. In comparison with 5.12 log10 IU/mL with TDF, the decrease in HBV DNA levels from baseline at week 24 were 5.40, 5.34, 5.33, and 5.40 log10 IU/mL with dosages of 30, 45, 60, and 75 mg of Pradefovir, respectively, as shown in Figure 1:
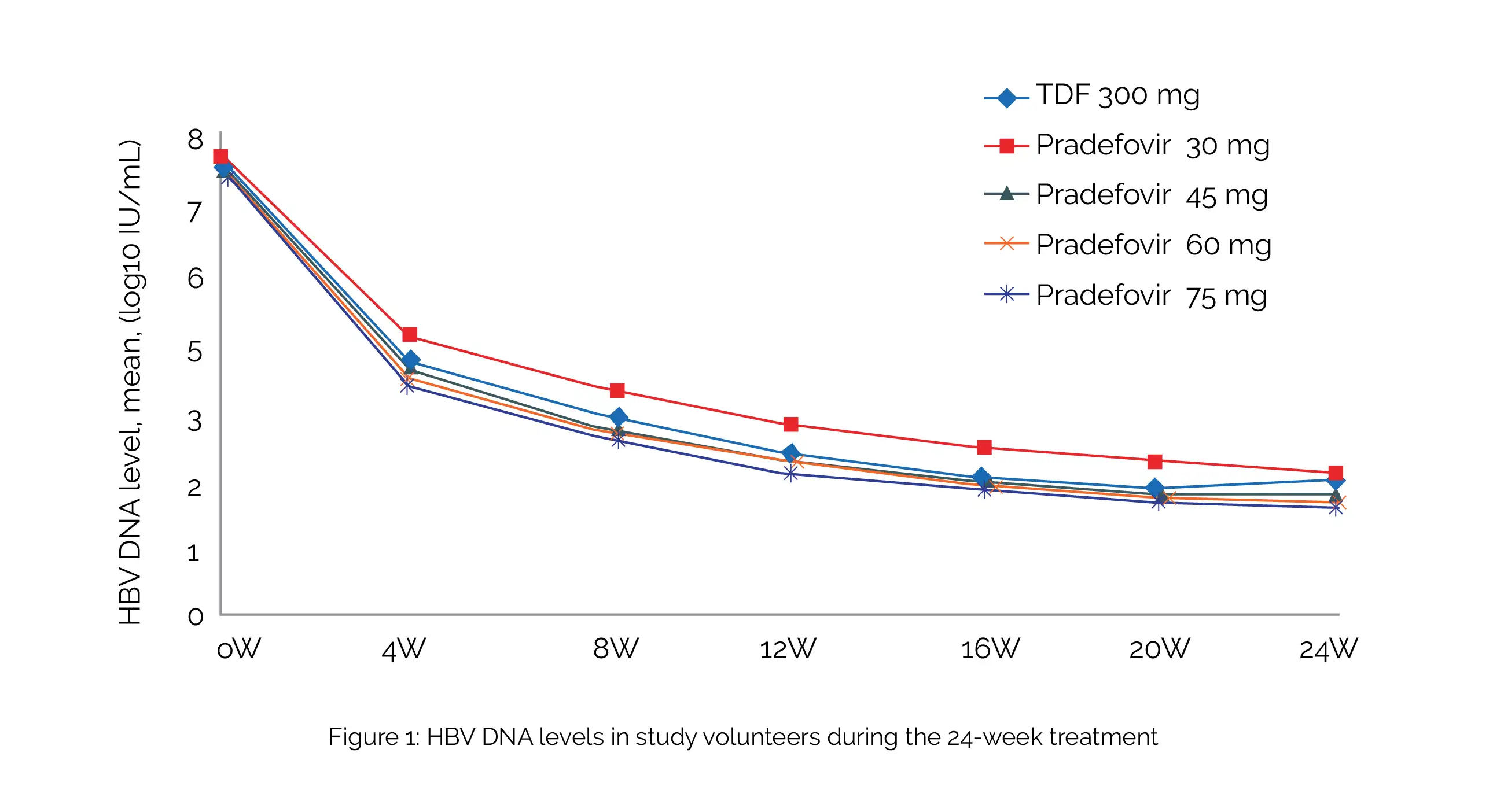
More volunteers who were treated with 45, 60, or 75 mg of Pradefovir experienced HBeAg loss (12%, 6%, and 9% vs 3%) compared to TDF-treated volunteers. While serum phosphate levels were similar across all groups, the TDF group showed a more remarkable rise in serum creatinine than the Pradefovir 30- and 45-mg groups. Side effects were mostly mild (grade 1).
No significant side effects associated with the therapy were recorded. Compared to those in the TDF group, the side effects and abnormal laboratory results were similar. Hence, Pradefovir (liver-targeted prodrug of adefovir) is a valuable nucleoside/nucleotide analogue for treating chronic hepatitis B.
Clinical Infectious Diseases
Pradefovir Treatment in Patients With Chronic Hepatitis B: Week 24 Results From a Multicenter, Double-Blind, Randomized, Noninferiority, Phase 2 Trial
Yanhang Gao et al.
Comments (0)