Categories
Change Password!
Reset Password!


Taking Budesonide in foam twice daily is more effective than once daily for early symptom resolution and better endoscopic remission in ulcerative colitis-affected patients.
Rapid relief from ulcerative colitis (UC) symptoms is crucial. A recent post hoc analysis published in the “Journal of Gastroenterology and Hepatology” explored how different dosing schedules of Budesonide rectal foam impact symptom resolution and endoscopic remission. Data from phase 2 and phase 3 trials of Budesonide foam (administered once or twice daily) and placebo were analyzed. The study involved 55 patients taking Budesonide once daily, 120 patients taking it twice daily and 116 patients in the placebo group.
The study assessed outcomes based on early (rectal bleeding subscore [RBS] 0 from week 2 to week 6), delayed (RBS 0 at week 6), and non-responders (RBS > 0 at week 6). At Week 6, 45.3% of patients on twice-daily (BID) Budesonide and 32.1% on once-daily (QD) Budesonide achieved an early response, compared to 12.8% in the placebo group. Table 1 depicts the complete endoscopic remission rate (Mayo endoscopic score [MES] value = 0) and endoscopic remission rate (MES value = 0 or 1) among BID recipients, categorized by responder status groups:
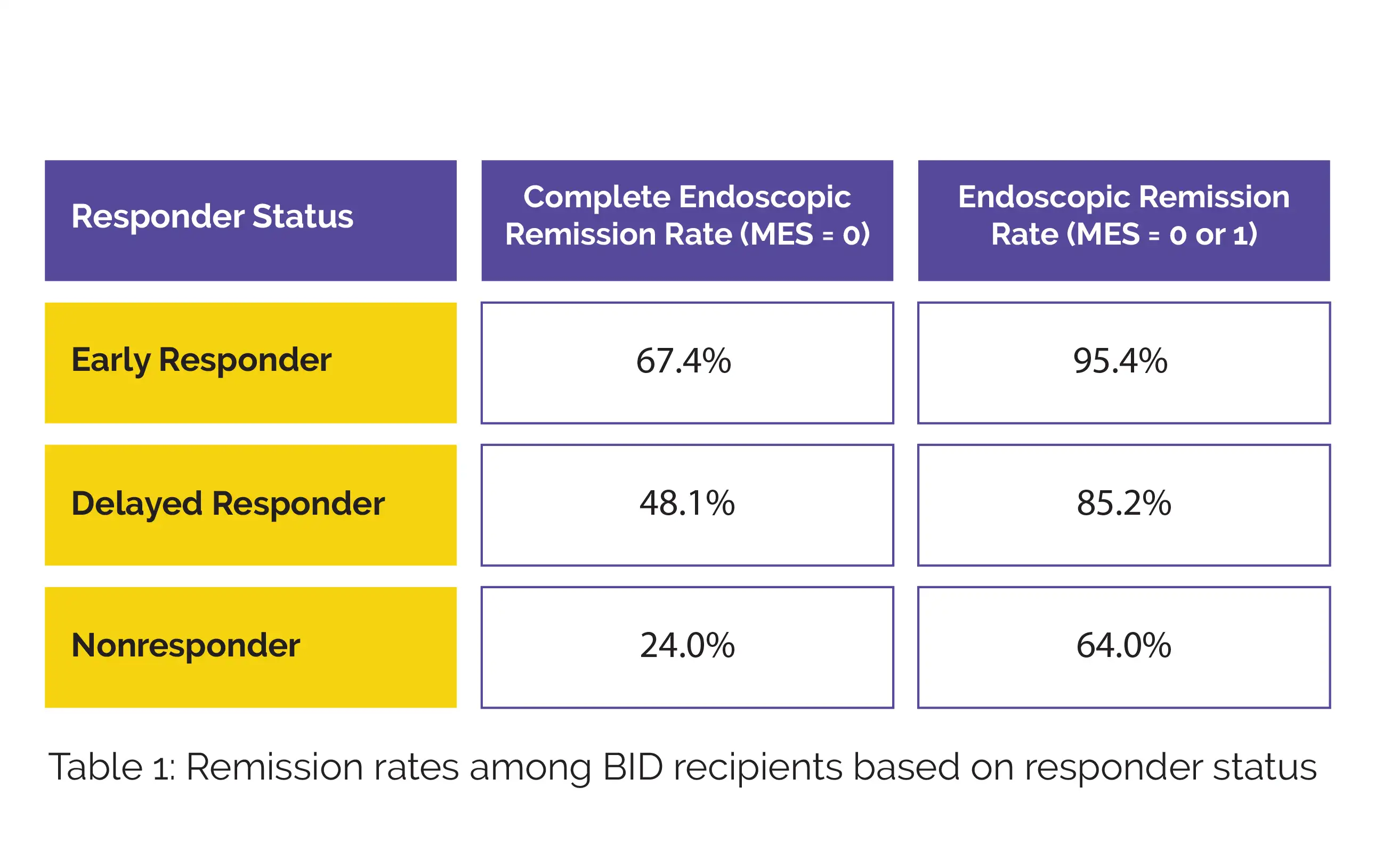
Most early responders attained endoscopic remission by week 6, regardless of the administration regimen. Early responders also had the longest cumulative non-relapse period among the groups (P = 0.07). The study concluded that twice-daily Budesonide foam is more effective than once-daily dosing in achieving early symptom relief and endoscopic remission in UC patients, leading to better long-term outcomes after treatment ends.
Journal of Gastroenterology and Hepatology
Impact of twice-daily budesonide foam administration on early clinical response and endoscopic remission in patients with ulcerative colitis: a post hoc analysis
Kenji Watanabe et. al.
Comments (0)