Categories
Change Password!
Reset Password!


Intervention with ubrogepant is beneficial when used in combination with onabotulinumtoxinA in migraine patients.
The results from the COURAGE study revealed that ubrogepant (oral calcitonin gene-related peptide [CGRP] receptor antagonist) aids in effective management of migraine when combined with onabotulinumtoxinA, an anti-CGRP monoclonal antibody (mAb). Aubrey Manack Adams et al. aimed to determine real-world efficacy of ubrogepant when used in combination with an anti-CGRP mAb preventive for acute treatment of migraine.
In this prospective, observational study, the collection of data was done using Migraine Buddy application. Eligible adult patients reported ≥3 migraine attacks in the last 30 days, had treated ≥3 former attacks with ubrogepant, and were concomitantly receiving an onabotulinumtoxinA, or both to prevent migraine. For thirty days, the patients continued the use of ubrogepant to manage attacks of any pain intensity. The meaningful pain relief (MPR) and return to normal function (RNF) post-dosing were the self-reported evaluations.
The outcomes from the first ubrogepant-treated attack and the first ten ubrogepant-treated attacks in the ubrogepant+CGRP mAb group were reported. Overall, 245 subjects reported utilizing ubrogepant as an acute intervention with an anti-CGRP mAb, with 1153 ubrogepant-treated attacks incorporated in the evaluation. For the first ubrogepant-treated attack and the 10 ubrogepant-treated attacks, the percentage of volunteers attaining MPR and RNF at 2-hours and 4-hours post-dose, is shown in Table 1:
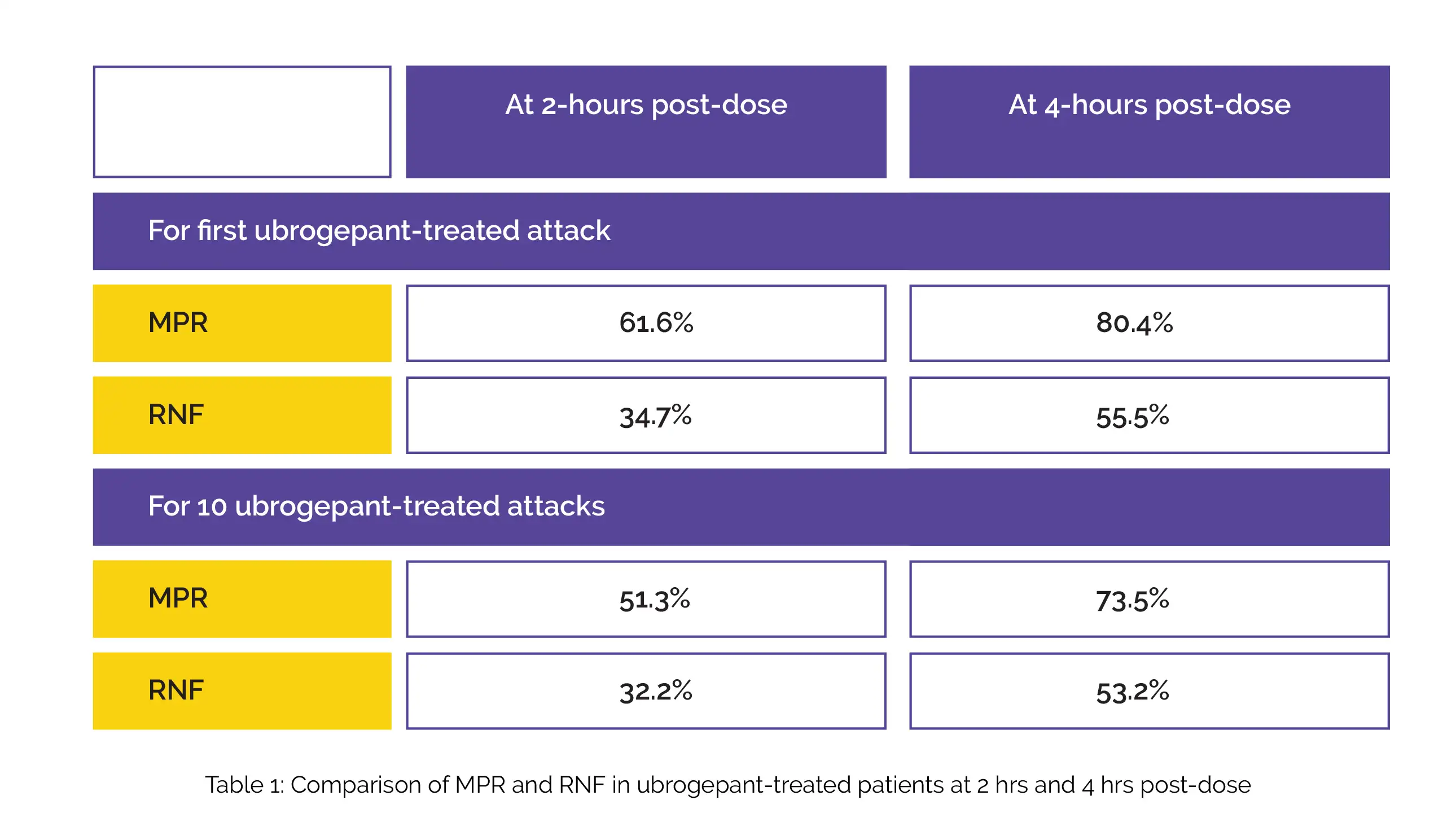
In clinical practice, the combination of ubrogepant and onabotulinumtoxinA is valuable and may assist physicians to optimize migraine management.
Neurology
Real-World Effectiveness of Ubrogepant for the Acute Treatment of Migraine When Used in Combination With an Anti-Calcitonin Gene–Related Peptide Monoclonal Antibody Preventive: Results From the COURAGE Study (P10-2.003)
Aubrey Manack Adams et al.
Comments (0)