Categories
Change Password!
Reset Password!


Ubrogepant enhances the quality of life for individuals suffering from migraine by effectively managing symptoms during the critical prodromal phase.
The analysis of the PRODROME trial revealed that Ubrogepant 100 mg, taken during the prodrome phase of migraine, drastically improved the patients' ability to function normally, displayed a notable decrease in activity limitations within 24 hours, and enhanced satisfaction with the treatment compared to placebo.
Ubrogepant, a calcitonin gene-related peptide receptor antagonist, is approved for the acute therapy of migraine. This latest evaluation by Richard B Lipton and colleagues focused on the patient-reported outcomes associated with Ubrogepant therapy. Adults who suffered from 2 to 8 migraine attacks per month, characterized by moderate to severe headache pain were included. Eligible volunteers treated 2 qualifying prodrome events, which were migraine attacks with early warning signs, where they were sure a headache would start within 1–6 hours. They were allocated to receive either a placebo followed by Ubrogepant or vice versa.
Out of the total 518 participants, 477 were retained in the revised intent-to-treat analysis. People treated with Ubrogepant 100 mg exhibited considerably better functioning over the course of 24 hours, with an odds ratio of 1.66. Notably, as soon as 2 hours post-treatment, a higher proportion of Ubrogepant recipients reported being “able to function normally” than the placebo group (Figure 1):
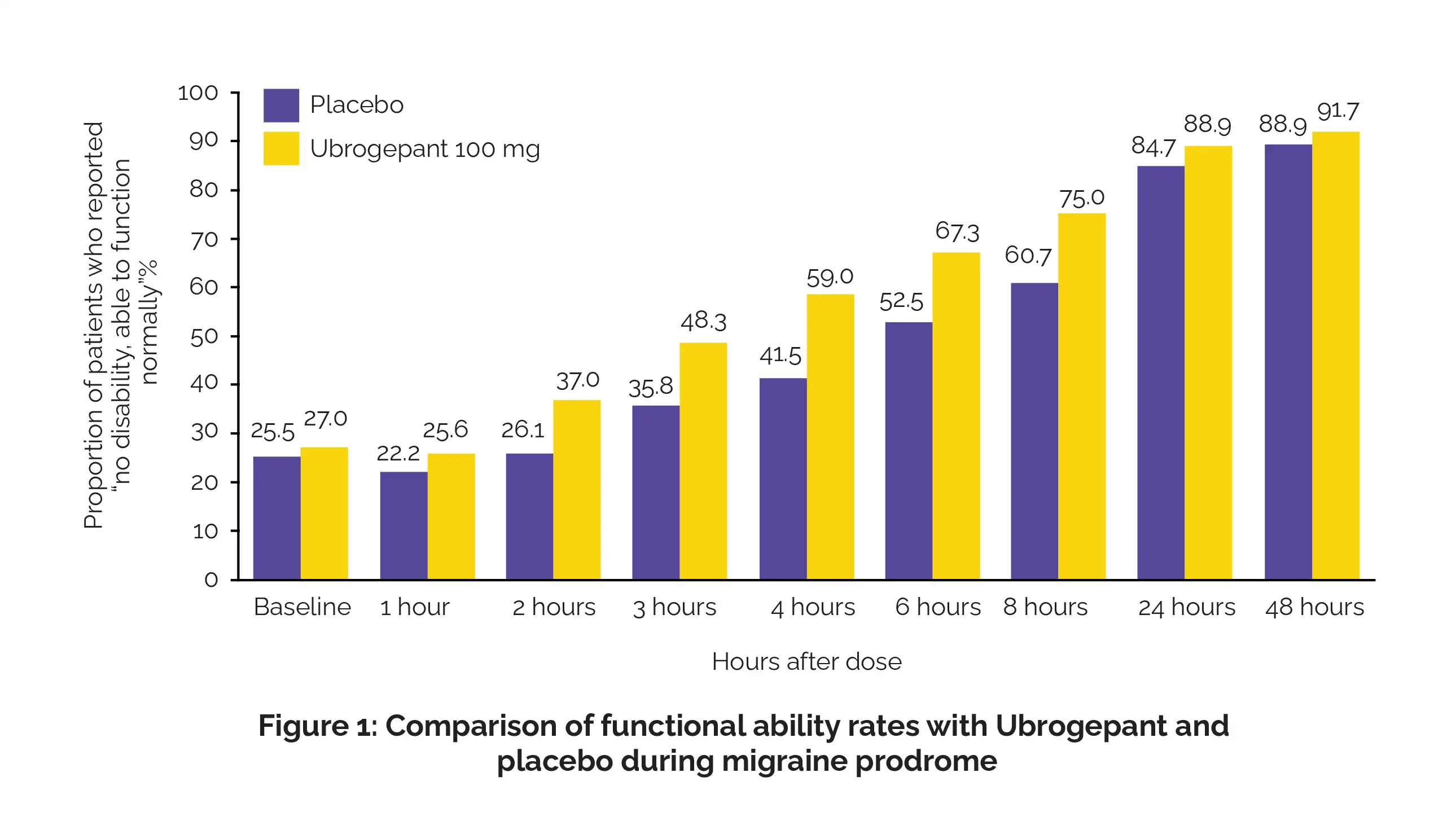
Furthermore, Ubrogepant led to a 2.07-fold increase in reducing activity limitations over the same period. Satisfaction rates were also markedly higher, with participants expressing greater contentment with the medication at both 8- and 24-hours after taking the intended dose (Figure 2):
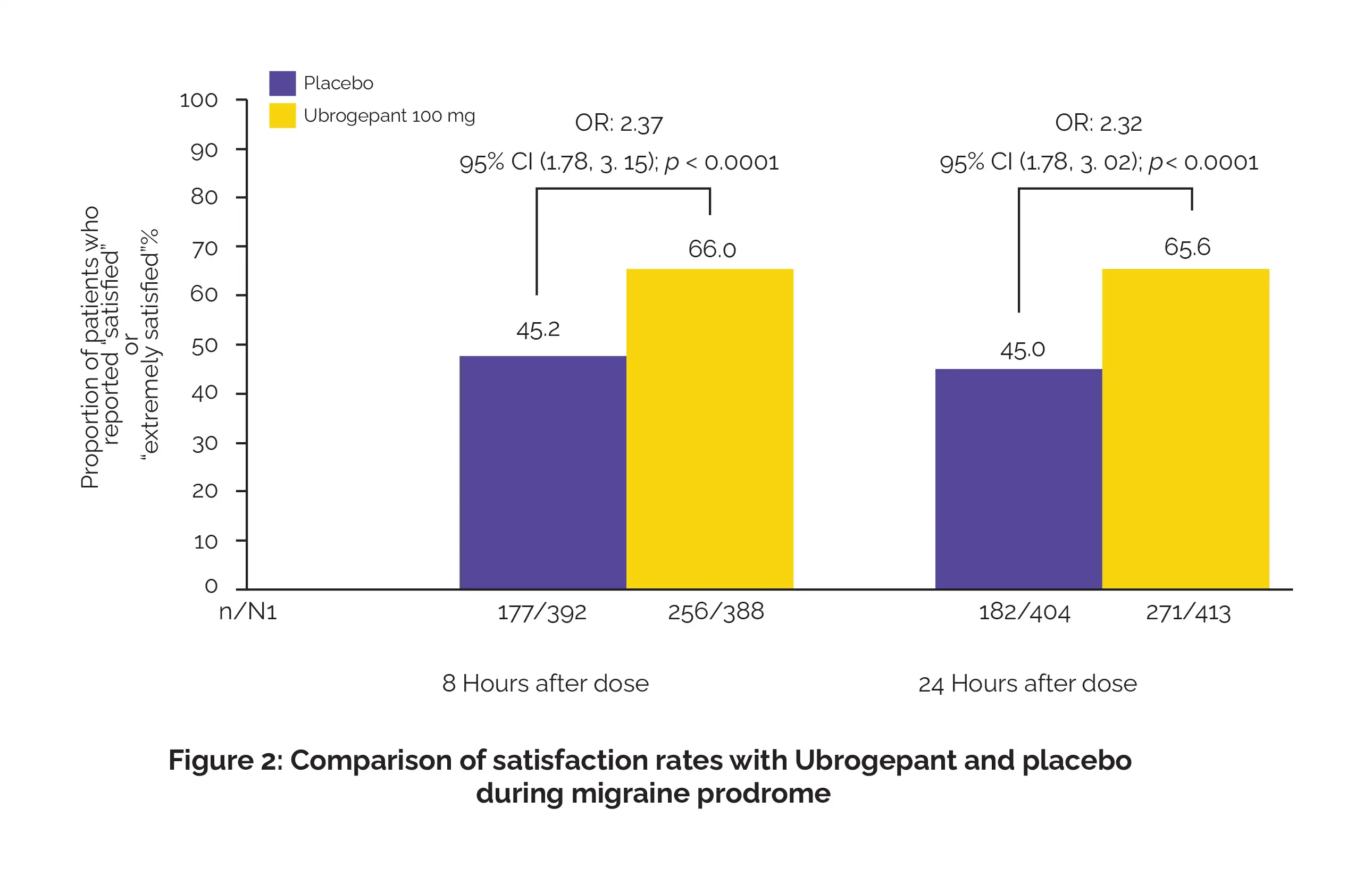
To sum up, Ubrogepant therapy not only proves effective, safe, and well-tolerated for migraines during prodrome but also revitalizes functionality for those affected.
Neurology
Effect of Ubrogepant on Patient-Reported Outcomes When Administered During the Migraine Prodrome: Results From the Randomized PRODROME Trial
Richard B Lipton et al.
Comments (0)