Categories
Change Password!
Reset Password!


For treating psoriatic arthritis, upadacitinib was found safe and effective when used both as monotherapy and combination therapy.
According to a recent study published in the Journal 'Rheumatology', the efficacy and safety of upadacitinib were comparable with non-biologic disease-modifying anti-rheumatic drugs (nbDMARDs) among patients with psoriatic arthritis (PsA).
This study was intended to assess the effectiveness and safety of upadacitinib as a monotherapy or combination regimen with non-biologic DMARDs in PsA patients. People with intolerance and unsatisfactory response to ≥ 1 nbDMARD or ≥ 1 biologic DMARD were administered with upadacitinib 15 mg once daily (QD) or 30 mg QD as monotherapy or in combination with ≤2 nbDMARDs and placebo for twenty-four weeks.
The efficacy endpoints of the study incorporated an assessment of minimal disease activity, Psoriasis Area and Severity Index responses, American College of Rheumatology responses (ACR), and substantial improvement in the Health Assessment Questionnaire-Disability Index. Adverse events were also assessed.
A total of 1916 patients have enrolled among which 70% of patients received combination therapy and 30% of patients received monotherapy. At week 12, the placebo-subtracted treatment effects for ACR20 were estimated to be as follows (Table 1):
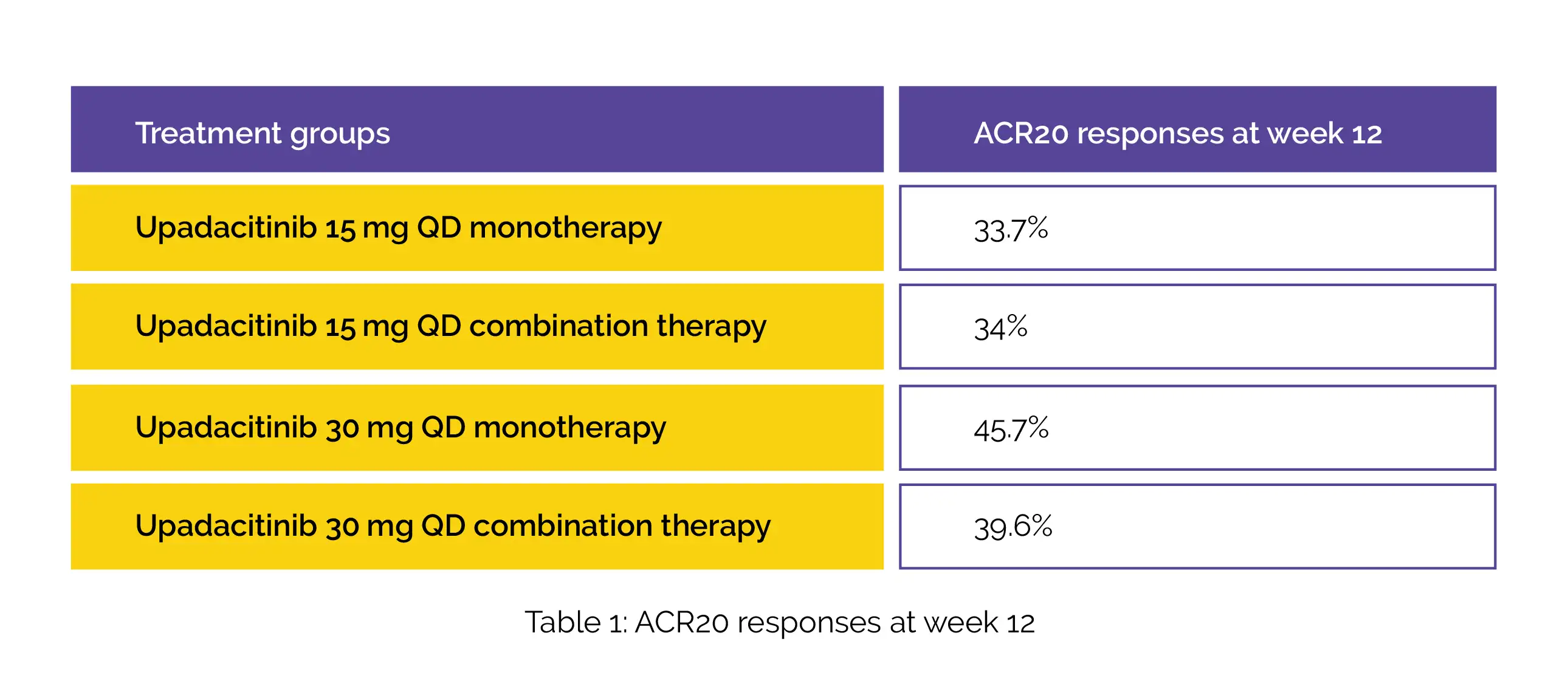
The treatment effects were consistent between combination therapy and monotherapy for all the other study outcomes. The frequency of noxious events was found to be comparable for both upadacitinib monotherapy and combination therapy. However, creatine phosphokinase elevation and hepatic disorders were more common among patients administered with combination therapy compared to patients administered with monotherapy.
These study findings supported the use of upadacitinib monotherapy and in combination with nbDMARDs in PsA. However, upadacitinib monotherapy can be effective among PsA patients who are unable to tolerate higher doses or have contraindications to methotrexate.
Rheumatology
Upadacitinib as monotherapy and in combination with non-biologic disease-modifying anti rheumatic drugs for psoriatic arthritis
Peter Nash et al.
Comments (0)