Categories
Change Password!
Reset Password!


The control of coronavirus outbreak has been quite challenging owing to high transmission rates from asymptomatic people, the emergence of several SARS-CoV-2 variants of concern, and the highly varying incubation period (ranging from 2 to 14 days).
Asymptomatic COVID-19 positive people living with an infected household contact, when treated with the combination of imdevimab and casirivimab vs. placebo substantially minimized the occurrence of symptomatic COVID-19 over 28 days.
The control of coronavirus outbreak has been quite challenging owing to high transmission rates from asymptomatic people, the emergence of several SARS-CoV-2 variants of concern, and the highly varying incubation period (ranging from 2 to 14 days). Due to immune-evading variants of concern, poor immune responses to vaccination in certain risk groups, and limited vaccine uptake in some areas, people still are at risk of getting infected and developing severe COVID-19 infection and longer-term problems (despite the availability of effective vaccines).
Complementary approaches like monoclonal antibodies against SARS-CoV-2 are required for non-vaccinated people. The human sequence monoclonal antibodies like casirivimab and imdevimab block the entry of the virus by binding nonoverlapping epitopes on the spike protein receptor-binding domain of SARS-CoV-2. Casirivimab-Imdevimab combination lowers the risk of development of treatment-elicited coronavirus variants.
Furthermore, the neutralization potency is retained in vitro against the already existing variants of concern including delta ( B.1.617.2), alpha ( B.1.1.7), gamma (P.1) and beta ( B.1.351). Treatment with casirivimab plus imdevimab is efficient to prevent infection in close-contact settings, and treat COVID-19 outpatients. Currently, it is approved in the United States under an emergency use authorization for management of mild-to-moderate coronavirus disease and for postexposure prophylaxis in few people.
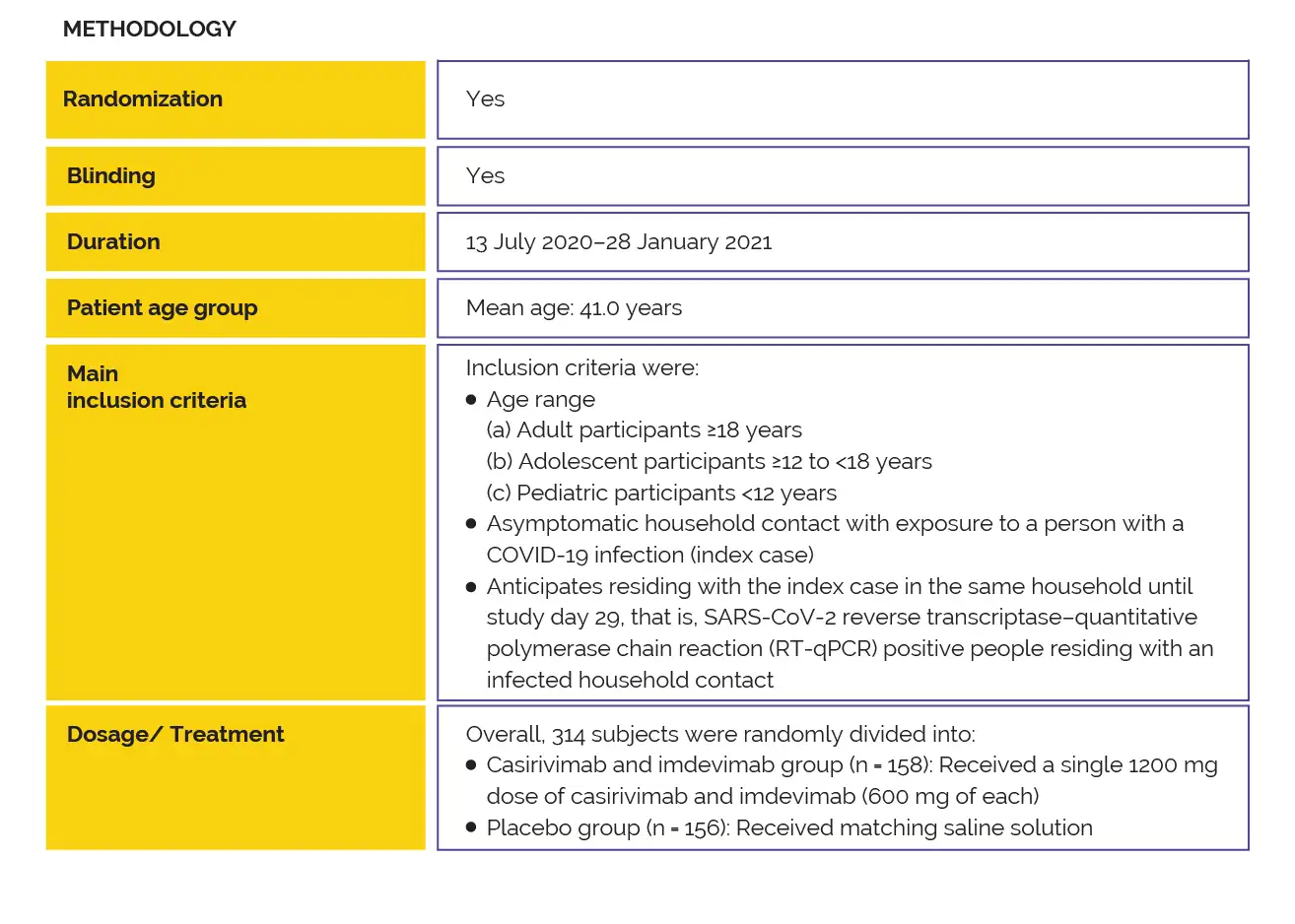
To explore if subcutaneous administration of casirivimab-imdevimab [1200 mg (600 mg of each antibody)] prevent coronavirus infection in asymptomatic household contacts of coronavirus-infected people, a two-part, randomized clinical phase III trial was performed in this high-risk setting. In uninfected close contacts, casirivimab-imdevimab profoundly prevented symptomatic coronavirus infection when compared to placebo (81.4% decrease in risk) in the first part of the trial.
This report elucidates the findings from Part B in which infected and asymptomatic close contacts were administered the combination of subcutaneous imdevimab and casirivimab (1200mg).
RATIONALE BEHIND RESEARCH
Till now, none of the prior studies has explored the impact of the subcutaneous casirivimab-imdevimab treatment on asymptomatic, infected close contacts of SARS-CoV-2-infected people. Thus, this randomized, double-blind, placebo-controlled study was carried out.
OBJECTIVE
A phase 3 clinical trial was performed to investigate the efficacy of subcutaneous casirivimab-imdevimab combination to prevent the advancement from early asymptomatic to symptomatic coronavirus infection in asymptomatic, infected household contacts of infected people.
Study outcomes
Outcomes
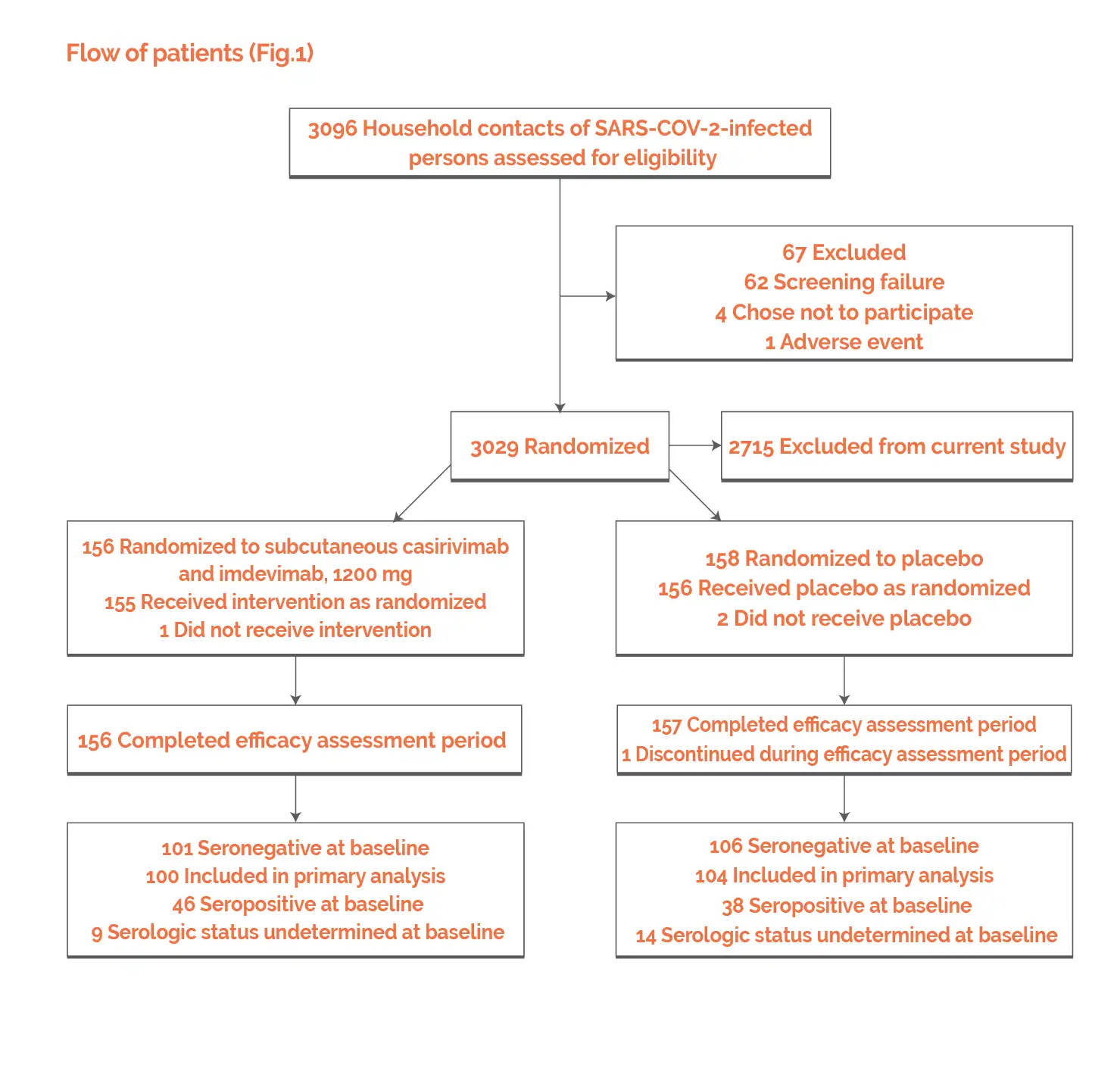
Baseline: There were no vital differences reported at baseline.
Study outcomes
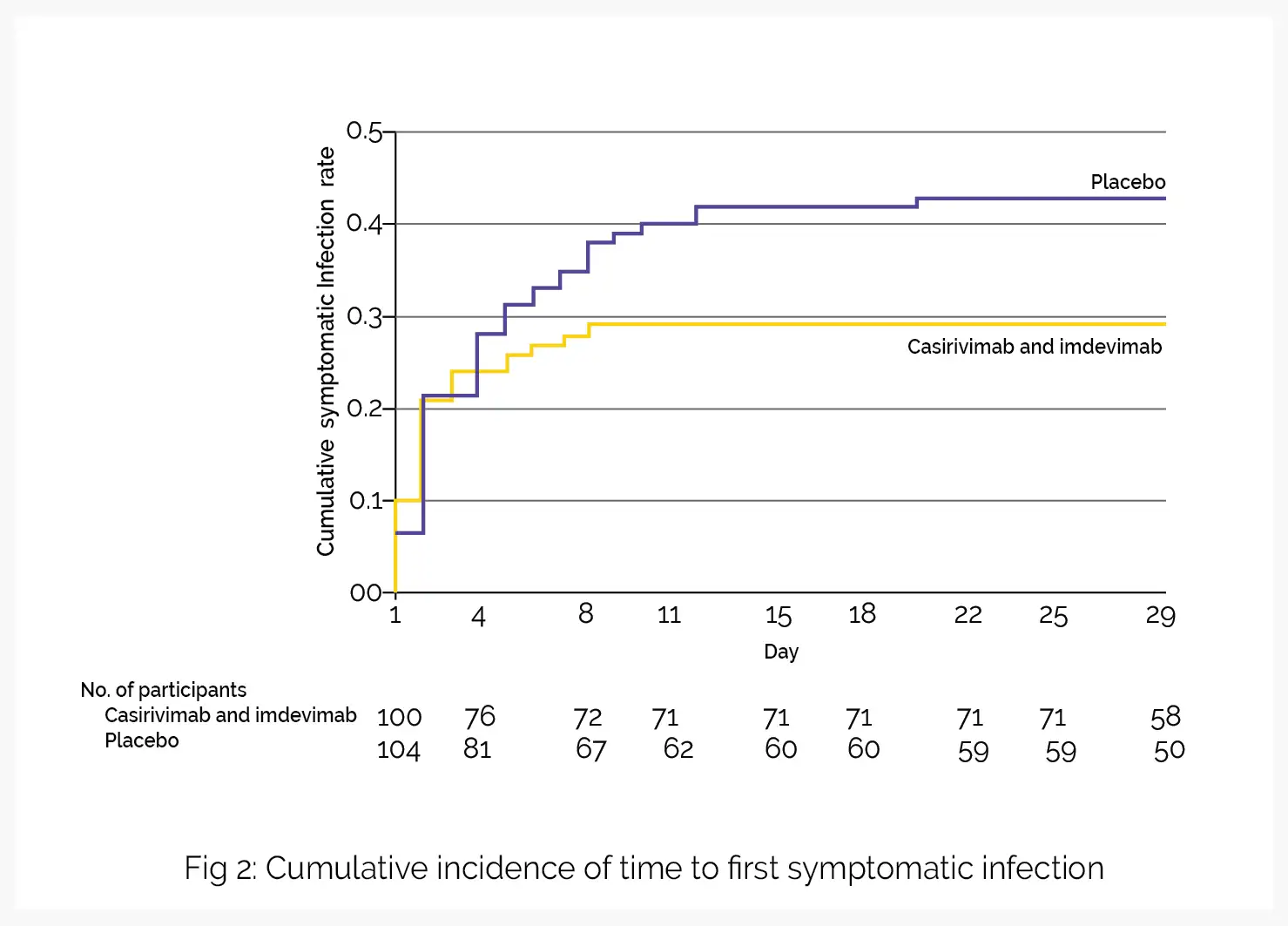
In part A of the study, subcutaneous administration of imdevimab and casirivimab aided in the prevention of asymptomatic and symptomatic coronavirus disease in uninfected people residing with an infected household contact. Part B of the study explored the influence of casirivimab plus imdevimab in people with early asymptomatic infection in the similar household contact setting. Compared to placebo, casirivimab and imdevimab lowered the incidence of symptomatic SARS-CoV-2 infection over 28 days.
The secondary endpoints favored casirivimab-imdevimab treatment over placebo in the duration of symptomatic infection, duration of RT-qPCR–detectable virus in the nasopharynx, and percentage of individuals with COVID-19–linked hospitalizations or emergency department visits. Decline in the development of symptomatic infection and in other results as noted would be of promising clinical significance to utilize monoclonal antibodies for the early management of coronavirus disease.
A post hoc assessment that investigated primary endpoint (development of symptomatic from asymptomatic SARS-CoV-2 infection) when symptoms initiated three days or longer post-therapy implied the advantage of casirivimab and imdevimab in lowering the incidence of symptomatic COVID-19 infection. However, this result must be considered hypothesis-generating. A prespecified exploratory assessment explored the major outcome in all patients irrespective of baseline serostatus.
This evaluation revealed that regardless of serostatus, the use of casirivimab-imdevimab decreased the succession to symptomatic COVID-19 from asymptomatic infection in the overall population. For guiding decisions in the clinic to prevent SARS-CoV-2 infection in exposed people, point-of-care serology tests might have a confined utility. The findings from a phase III trial involving people suffering from coronavirus disease demonstrated that intravenous administration of casirivimab and imdevimab (1200 mg) lowered the risk of SARS-CoV-2-linked mortality or hospital admission, decreased the time to resolution of symptoms, and minimized viral load faster when compared to placebo.
The emergency use authorization permits subcutaneous administration of 1200mg as a substitute route of administration when the intravenous infusion is not feasible and can elicit therapy delay. According to the current study, investigation of the secondary outcome of COVID-19-linked medically attended visits during the 28-day efficacy evaluation period stated that six placebo-treated patients had an emergency department visit or hospital admission while no patients getting subcutaneous imdevimab and casirivimab showed these events, in support of the use of 1200mg subcutaneous administration as a substitute route of administration for COVID-19 patients, as approved under current emergency use authorization.
In imdevimab and casirivimab studies, a higher percentage of people who got placebo reported one or more treatment-emergent adverse events (TEAE), with the difference attributed to an escalated number of SARS-CoV-2 associated events seen in that arm. After subcutaneous administration of the dose, the concentrations of each antibody in serum were found to be above the anticipated neutralization target concentration, based on preclinical and in vitro data, on the 1st day after dosing and throughout the 28-day efficacy evaluation period.
An examination of pharmacokinetic profiles after subcutaneous or intravenous single doses of imdevimab and casirivimab showed that although intravenously given casirivimab (600mg), and imdevimab (600mg) attained elevated drug concentrations at early time points, subcutaneously given casirivimab (600 mg), and imdevimab (600 mg) attained mean concentrations in serum after one day of administration of 22.1mg/L and 25.8mg/L, respectively. These day 1 levels were noted to be above the estimated target dose for virus neutralization (20 mg/L).
Casirivimab-imdevimab combination prevented progression to symptomatic COVID-19 among recently exposed asymptomatic RT-qPCR positive people living with an infected household contact. Thus, such easy-to-administer therapies can be used for reducing viral carriage and preventing advancement from asymptomatic infection to symptomatic disease.
The Journal of the American Medical Association
Effect of Subcutaneous Casirivimab and Imdevimab Antibody Combination vs Placebo on Development of Symptomatic COVID-19 in Early Asymptomatic SARS-CoV-2 Infection: A Randomized Clinical Trial
Meagan P O'Brien et al.
Comments (0)