Categories
Change Password!
Reset Password!


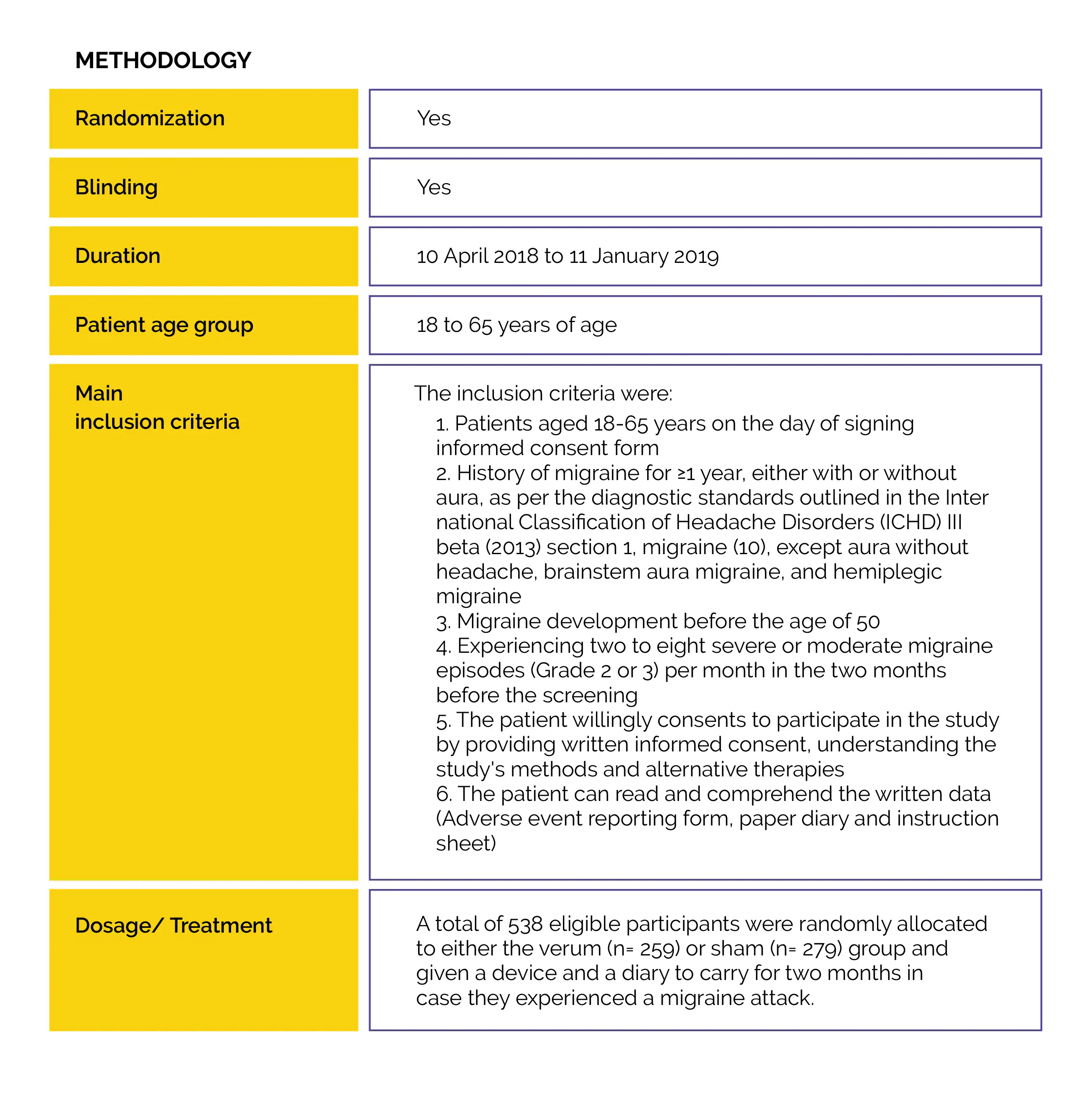
The World Health Organization (WHO) has listed migraine as the second biggest cause of disability in the world.
In an at-home setting, treatment with 2-hour External Trigeminal Nerve Stimulation (e-TNS) is an efficient, safe, non-pharmacological, and non-invasive option for the acute treatment of migraine headaches.
The World Health Organization (WHO) has listed migraine as the second biggest cause of disability in the world. It is one of the most prevalent neurological disorders. Currently, triptans, non-steroidal anti-inflammatory drugs (NSAIDs), lasmiditan, and gepants (rimegepant and ubrogepant) are the first-line acute therapies for migraine. Acute pharmacological migraine therapies may be contraindicated in individuals having cerebrovascular and/or cardiovascular illness, may have serious adverse effects, or be ineffective at relieving pain. As a result, around 40% of individuals with episodic migraines have unfulfilled treatment requirements. The overuse of acute medications in the treatment of migraine is another issue.
Regular usage of acute migraine drugs may raise the risk of medication overuse headache (MOH), migraine chronification, and adverse effects. Research has unveiled that the majority of individuals treat their migraine using non-pharmacological methods. For migraine sufferers who would avoid pharmaceutical therapies, have contraindications or intolerance to acute migraine interventions, or do not respond to pharmacologic acute migraine therapies, e-TNS is a non-invasive, non-pharmacological therapeutic choice.
There are a few hypothesised processes for how e-TNS works as a migraine therapy. It is commonly known that the trigeminovascular system plays a pivotal role in migraine. The first division of the trigeminal nerve includes the branch of the supraorbital nerve that is directly activated by e-TNS. According to fluorodeoxyglucose-positron emission tomography (FDG-PET) research, e-TNS may alter how some parts of the brain regulate pain.
Numerous studies have illustrated efficiency and safety of e-TNS for the acute treatment of migraine attacks, encompassing the sham-controlled Acute Migraine therapy with External Trigeminal Neurostimulation (ACME) trial. In the ACME experiment, e-TNS was used in the hospital setting, with a small sample size, and following a migraine that lasted for four hours before the intervention. The results of the ACME trial illustrated a tendency for longer treatment times to favor better relief of migraine.
RATIONALE BEHIND RESEARCH
There is no data available from a clinical study utilizing a comparable protocol design and outcomes in a real-world, at-home environment. Thus, a randomized study [Trial of e-TNS for the Acute Treatment of Migraine (TEAM)] was carried out.
OBJECTIVE
The safety and effectiveness of self-administered, 2-hour (two consecutive 1-hour) e-TNS therapy for migraine episodes with and without aura in an at-home situation were investigated in a Phase 3 double-blind, multicenter, prospective, sham-controlled trial.
Study outcomes
Pain freedom at 2 hours and remission of the most bothersome symptoms (MBS) associated with migraine at 2 hours after the start of e-TNS treatment for one qualifying migraine attack were the major endpoints ascertained.
The secondary outcomes were:
(a) Utilization of a rescue drug between 2-24 hours following the start of an e-TNS session
(b) Pain alleviation after two hours characterized as a decrease from a baseline moderate or severe migraine headache to a mild or no headache
(c) Sustained pain alleviation at 24 hours characterized as mild or no headache at 2 hours or mild or no headache at 24 hours following the start of the e-TNS session without using anti-migraine medication throughout those 24 hours
(d) Sustained pain freedom characterized as pain freedom at two hours and pain freedom at 24 hours without the usage of an anti-migraine drug
(e) Resolution of any migraine-related symptom two hours after the start of e-TNS therapy
Outcomes
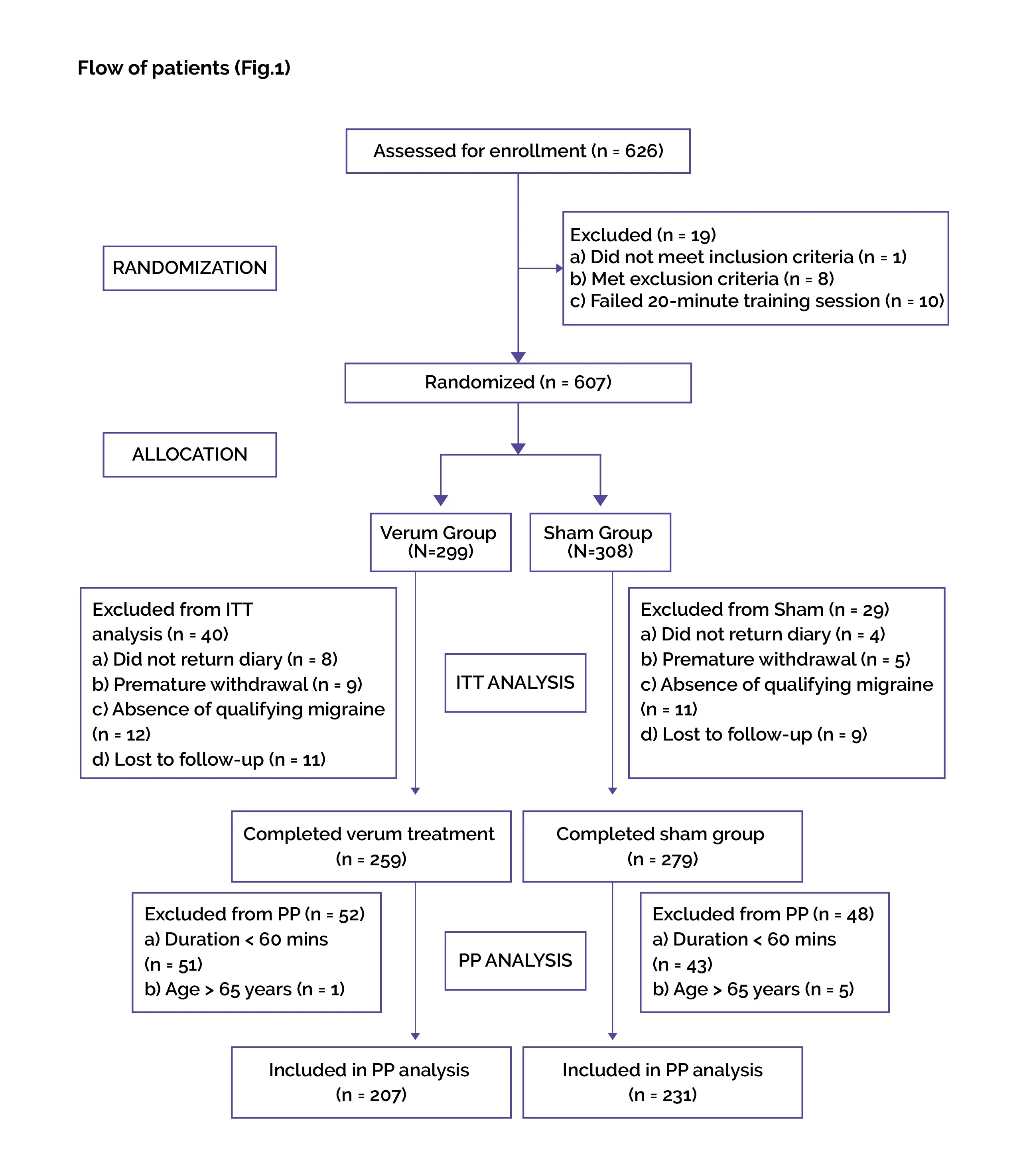
Baseline: There were no vital differences reported at baseline.
Study outcomes
##IMG4#
The prospective, multicenter TEAM study offers the first Phase 3 evidence establishing the safety and effectiveness of patient-administered, 2-hour e-TNS therapy for the management of migraine with and without aura in the out-of-hospital environment. Previous trials on the use of e-TNS to treat acute migraine attacks revealed that a lengthier course of treatment might lead to better pain freedom and long-lasting migraine relief.
When compared to sham therapy, 2-hour e-TNS treatment for acute migraine showed substantially greater rates of migraine pain relief at 2 hours and remission of migraine-related MBS. As contrasted to the sham group, 2-hour e-TNS for relieving migraine episodes produced persistent pain relief at 24 h, sustained pain freedom, resolution of all migraine-related symptoms, and a higher rate of pain relief at 2 h.
The outcomes supported earlier assumptions that e-TNS treatment duration has a profound role in attaining optimal pain management. In the TEAM trial, fewer subjects reported being pain-free after 2-h of e-TNS treatment than had been prior reported in an open-label, non-sham controlled, Rochester trial (25.5 vs. 35.6%, respectively). The percentage of patients experiencing MBS resolution (56.4% vs. 60.4%), sustained 24-h pain freedom (23.2% vs. 25%), sustained pain alleviation at 2-h (69.5% vs. 70.8%), and absence of all migraine-associated symptoms (42.5 vs. 48.8%) were similar between the precedent open-label study and the TEAM study.
The results of this study showed that 2-h e-TNS is effective in treating acute migraine with and without aura. Between the verum and sham groups, there were no appreciable differences in the usage of acute migraine medications at 2 and 24 hours. This finding was in line with the findings of the ACME study. Even though e-TNS has been found to be a safe, non-invasive, and effective substitute for acute migraine medications, the consistency of these findings may favor using e-TNS as an adjunct or supplemental therapy in the at-home situation.
To fully understand the practical function of e-TNS in a stratified approach to acute migraine therapy, more trials are required. It is still uncertain how much sham stimulation contributed to a partial therapy effect compared to a true placebo effect, despite the fact that the stimulation frequency between the sham and verum differed in this trial. Comparable sham stimulation parameters were employed in earlier e-TNS trials proving its effectiveness. A future randomized, self-control longitudinal trial that permits to treat several migraine episodes over the course of three to four months would be beneficial for determining how consistently acute e-TNS therapy for migraine attacks works.
In both groups, migraine sufferers with aura had a more powerful response to e-TNS stimulation. Between the verum and sham groups, no discernible differences in responses were found. Mechanism of these outcomes is not clear. Patients who experience migraine aura may have early warning signs of migraine onset, enabling quicker diagnosis and treatment of migraine. Early migraine identification and treatment are linked to a higher chance of successful migraine therapy, according to the trials of acute migraine medicines.
The e-TNS initiation time from migraine commencement was not procured in the TEAM trial. To distinguish between the efficacy of e-TNS treatment early vs. late in the trajectory of migraine attack, more trials are required. No major adverse events were witnessed. All device and non-device associated noxious events were minor and fully reversible without the intervention at the end of the therapy. The most frequent adverse side effect, discomfort and forehead paresthesia, was in line with previous study findings.
In contrast to the sham group, more patients in the verum group reported device-related adverse effects. Although only a small percentage of patients (3.9%) had this adverse event, this outcome would suggest that 2-h e-TNS therapy is linked to a greater prevalence of forehead paresthesia. The success of e-TNS in relieving migraine depends on patient tolerance and the occurrence of side effects. In a previous assessment of 2313 subjects utilizing e-TNS, patients who were dissatisfied with their medication had a greater risk of adverse events.
In this study, e-TNS was discontinued by 2% of participants because of adverse events, while adverse events were experienced by almost 9% of subjects with suboptimal compliance. Ten volunteers in the TEAM trial were not included in the randomization process because they could not tolerate the nociceptive test. Multivariate analysis showed that contrasted to patients without adverse events, individuals reporting adverse events were 1.33 times more probably to utilize acute migraine medicine between 2 to 24 hours.
These results underline the significance of patient tolerability of stimulation measures for compliance and success of migraine and migraine-related symptom response. The e-TNS device offers a "stabilization feature" that, in clinical settings, permits patients to stabilize or plateau the stimulation intensity within the initial fourteen minutes if paresthesia becomes excessively strong. This characteristic may lessen the possible intolerance to stimulation at greater intensities, especially during initial therapy.
Therefore, it is essential that patients are informed about the purpose and use of this feature in order to maximize adherence and tolerance to the therapy. Concerns about patient compliance may arise given the 2-hour treatment duration's practicality. Contrasted to a 2-hour therapy, compliance rates were greater for a 60-minute treatment.
This finding could point to a balance between the ideal duration of neuromodulation therapy and the desired level of pain alleviation. The TEAM study's compliance rates and median length of treatment were comparable to those of the earlier open-label trial, which shows that 2-hour e-TNS treatments are feasible and realistic substitutes for traditional medical treatments in a real-world setting.
For migraine management, e-TNS is effective, has a good safety profile, and is a useful alternative in an at-home setting.
Scientific Reports
Phase 3 randomized, double-blind, sham-controlled Trial of e-TNS for the Acute treatment of Migraine (TEAM)
Deena E. Kuruvilla et al.
Comments (0)