Categories
Change Password!
Reset Password!


Osteoarthritis, a chronic degenerative joint disorder, is marked by disability and eventually invalidating pain that results in seeking medical assistance.
Compared to usual care, duloxetine intervention leads to a significant reduction in neuropathic-like symptoms and improvement in pain, functional status, and quality of life in patients with end-stage knee osteoarthritis and central sensitization pain.
Osteoarthritis, a chronic degenerative joint disorder, is marked by disability and eventually invalidating pain that results in seeking medical assistance. In osteoarthritis people, the pain experience usually transitions from intermittent weight-bearing to ongoing and highly persistent chronic pain. Osteoarthritis treatment is aimed at pain alleviation for regaining quality of life and physical function. Few people do not experience satisfactory pain mitigation from the first-line treatment modalities such as acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs).
This ineffectiveness possibly emerges from osteoarthritis-mechanopathology and the biological response to mechanically elicited injury, that likely differs per individual. An alteration in the biochemical environment around joint structures and peripheral joint nociceptors is one pivotal biological response. This may lead to the hyperexcitability of peripheral and ultimately central nervous system (central sensitization).
Central sensitization is characterized as elevated responsiveness of nociceptive neurons in central nervous system and might incorporate raised responsiveness due to dysfunctioning of the endogenous pain control systems. The preoperative central sensitization combined with peripheral articular nerve alterations are accountable for joint-associated neuropathic-like symptoms like allodynia and hyperalgesia. Approximately 20–67% of knee osteoarthritis people and 20% of hip osteoarthritis people experience those symptoms.
Several studies state that these symptoms are strongly associated with basic pain intensity. The selective serotonin and norepinephrine reuptake inhibitor duloxetine appears to be efficacious to treat neuropathic pain conditions and chronic pain conditions in which one of the principal underlying pain mechanisms is central sensitization. Contrary to conventional osteoarthritis analgesics, duloxetine's mechanism of action appears to be linked with improvement of central pain control system by affecting serotonin and norepinephrine transporters (activating descending pain inhibitory system).
In people having knee osteoarthritis, the placebo-controlled randomized studies have demonstrated duloxetine's efficacy as a promising analgesic in the conservative therapeutic phase of osteoarthritis. But, there is a lack of far studies among hip osteoarthritis patients. Furthermore, all the previous knee osteoarthritis studies were placebo-controlled and hence did not incorporate a care-as-usual control situation. For enhancing external validity, pragmatic randomized trials are required.
Since the subgroups of osteoarthritis people can react distinctly to analgesic agents, selecting a predefined group of potential responders to the therapy (like people experiencing osteoarthritis pain with neuropathic features) – can even improve the outcomes and decrease number needed to treat.
RATIONALE BEHIND RESEARCH
No trial has investigated duloxetine versus usual care in the end-stage hip and knee osteoarthritis people with centralized pain. Thus, this pragmatic, enriched, multicenter trial was carried out.
OBJECTIVE
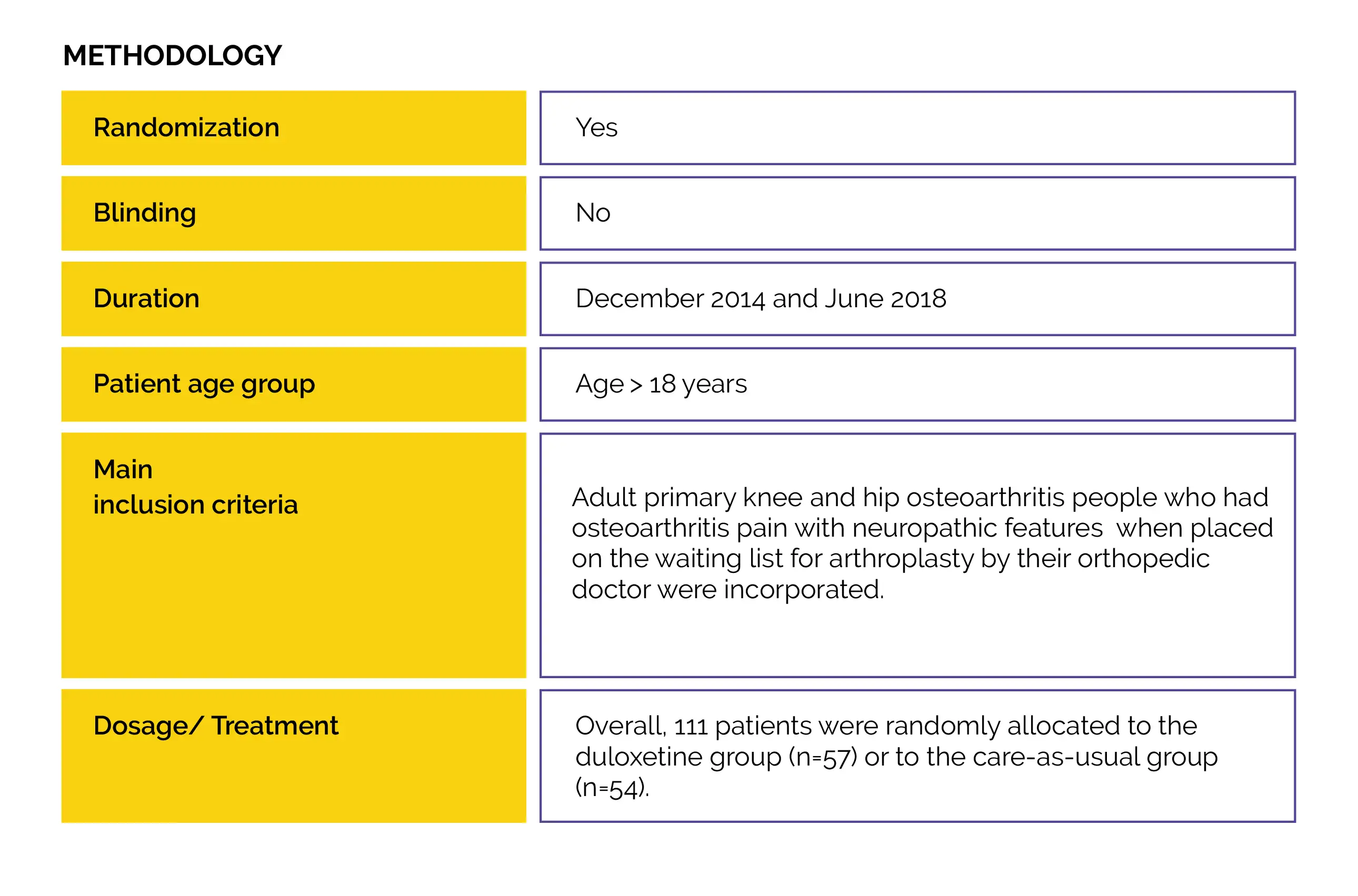
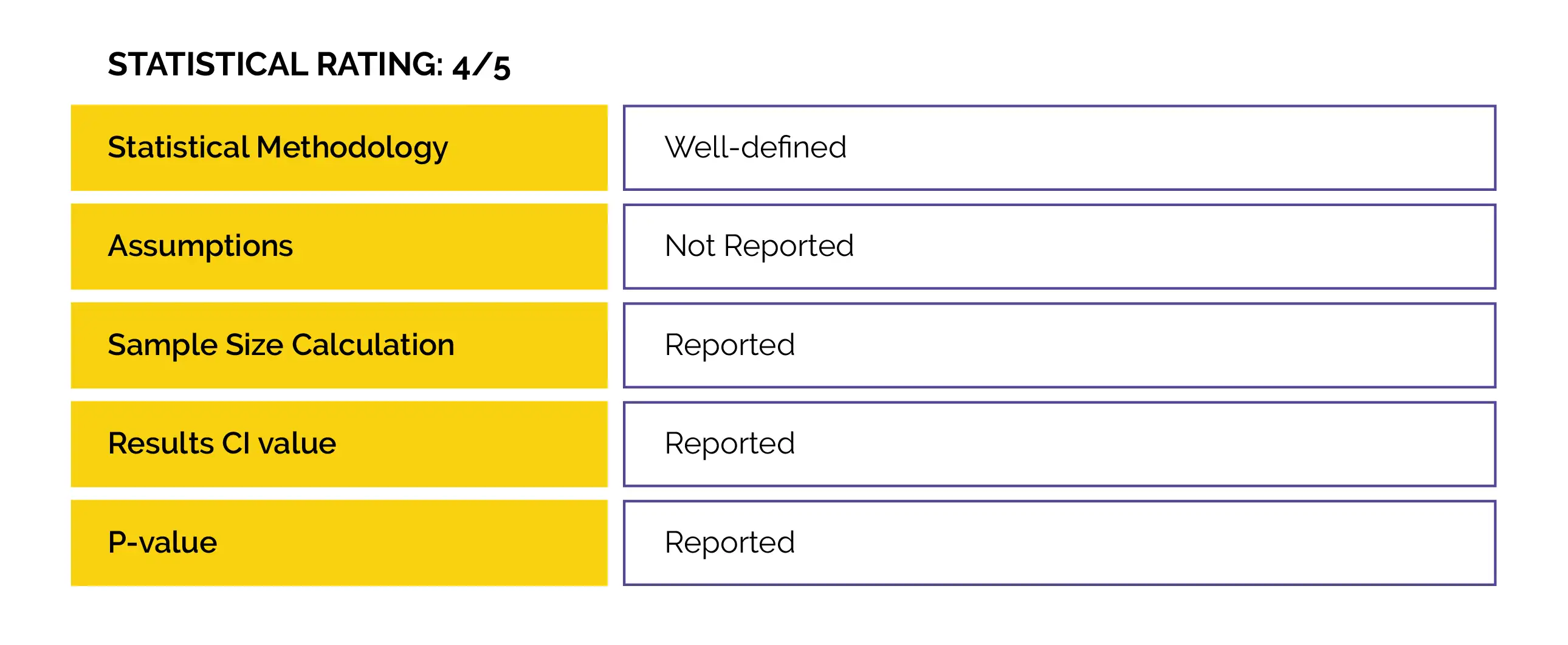
An open-label randomized controlled trial (Duloxetine in OsteoArthritis [DOA]) was performed to examine duloxetine vs. usual care effectiveness to decrease knee- or hip-associated pain in people with end-stage hip and knee osteoarthritis. Also, the impact on patient’s global impression of improvement, physical functioning, neuropathic-like symptoms, and pain sensitization were evaluated.
Study outcomes
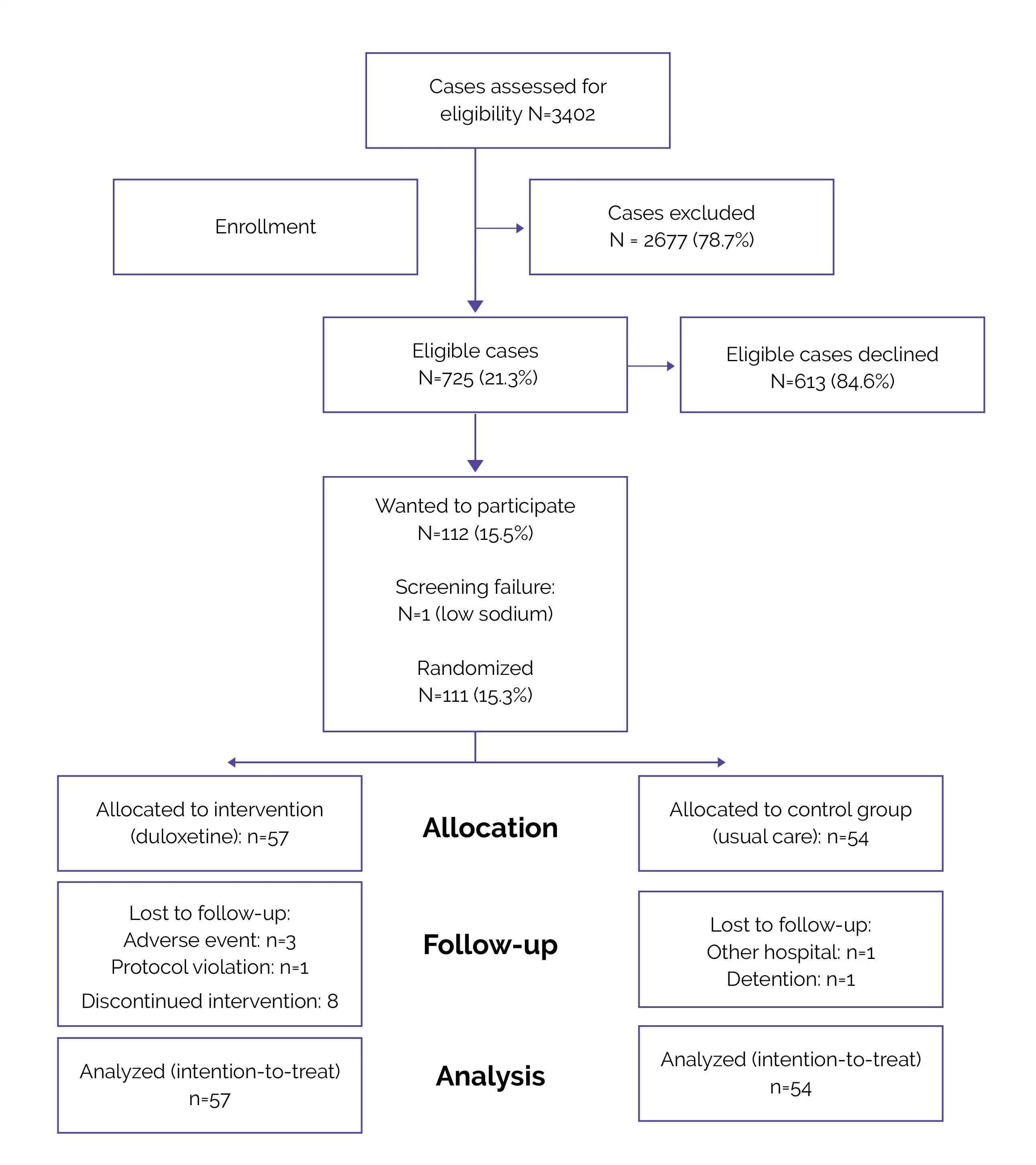
Flow of Patients (Fig.1)
Outcomes
Baseline: There were no pivotal differences reported at baseline.
Study outcomes
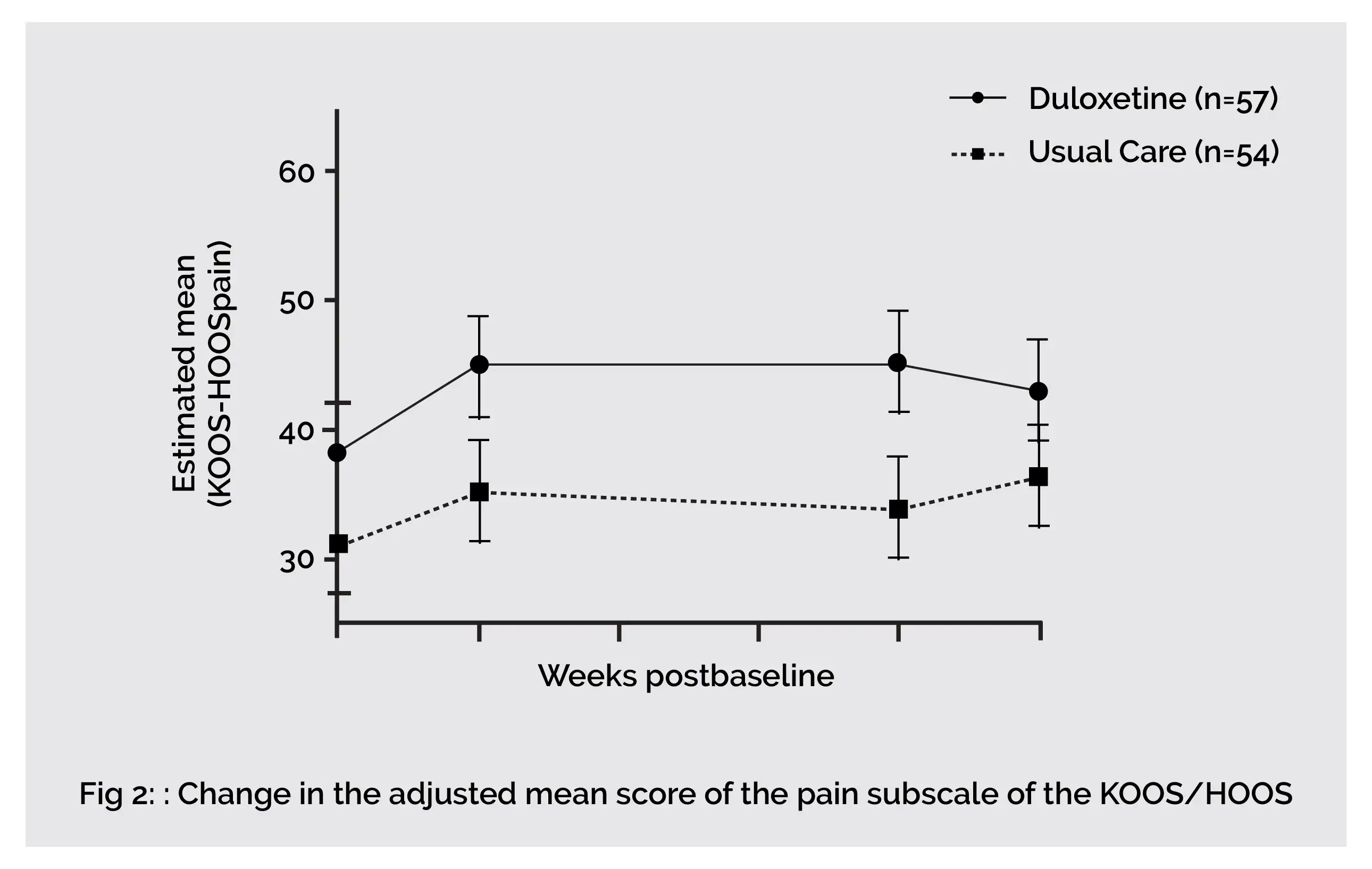
In end-stage knee and hip osteoarthritis people, an eight-week duloxetine intervention showed superior analgesic effects when compared to usual care. Compared to 0% in the usual care group, about 44% of people reported much to very much better following eight weeks of duloxetine use. Duloxetine exhibits a significant effect in improving joint associated function and pain (KOOS/HOOS Pain, Symptoms and Activities of Daily Living domain). However, end-stage hip osteoarthritis people are nonresponsive to duloxetine therapy.
An additional separate sub-assessment indicated that the reported effects in the total study group were described by reduction of pain and symptoms in people having knee osteoarthritis who used duloxetine therapy. Significant effects of duloxetine were only noted among knee osteoarthritis people. But, just like knee osteoarthritis people, hip osteoarthritis people did report subjective improvement (on the Patient Global Impression of Improvement [PGI-I]) in their symptoms.
The two-week tapering phase that incorporated two weeks on half the treatment phase dosage demonstrated reduced analgesic effects of duloxetine. In the study, none of the differences within the function and pain-associated measures on HOOS/KOOS attained significant threshold levels. The findings of the joint-specific sub-assessment demonstrated that the mean differences between the two groups were still statistically significant for all the outcome measures, including pain-related ones in people with osteoarthritis of the knee.
In both knee and hip osteoarthritis people, the perceived level of improvement following tapering (on PGI-I) did reduce from 43% to approximately 20%. Despite a few issues of heterogeneity, the outcomes reported in previous studies were comparable to this study. Previous studies reported that compared to the care-as-usual group, the duloxetine group exhibited superiority in terms of alleviation of osteoarthritis-associated pain. The current study also reported a similar percentage of people who stated that their joint complaints were much to very much better following treatment with duloxetine.
Regarding the effect of duloxetine intervention, the discrepancy between knee and hip osteoarthritis is clearly detectable. However, the study was not powered to detect prominent differences within the two osteoarthritis entities separately. Contrarily, almost all the differences in the knee osteoarthritis group were clinically meaningful. Regarding the occurrence of noteworthy differences between the two osteoarthritis entities, one factor can be that knee osteoarthritis people are highly centrally sensitized and hence are highly vulnerable to respond to a centrally acting agent such as duloxetine.
Notably, this can be due to improved pain perception and proprioception in knee osteoarthritis, that stimulates early symptomatic presentation. Consequently, knee osteoarthritis people report more long-lasting pain, that can lead to sensitization of peripheral and later the central nervous system. Another reason could be due to decreased bioavailability of duloxetine as a result of smoking. Smoking leads to a rise in the expression of CYP1A2, that is linked with a one-third drop in duloxetine's bioavailability.
Larger robust studies, adequately powered for each joint, are required for drawing conclusions. A larger study can offer better understanding of the effect of duloxetine in a subgroup of people who suffer from more neuropathic-like symptoms (raised Modified painDETECT [mPDQ] scores). Approximately all the people who used duloxetine reported treatment-emergent adverse events (94.7%). These were generally modest and principally present at the commencement of duloxetine therapy.
Another study by Wang et al. reported that with 60 mg/day duloxetine use for thirteen weeks, 60.8% of people had at least one treatment-emergent adverse event. The discontinuation rate due to adverse events showed consistency with the percentages reported in the literature, 16.3% vs 21.1% in the present study. The chief insight is that end-stage knee osteoarthritis people still have the ability to respond well to conservative duloxetine therapy.
Remarkable improvement in pain was already pronounced following two weeks of therapy. Due to this, it can be hypothesized that the costly and frequently performed joint-replacement surgery can be postponed in a subset of people, or could even no longer be required. Another novel insight is that it seems possible to desensitize knee osteoarthritis people preoperatively. Significant pain alleviation up to ten weeks was witnessed in individuals suffering from osteoarthritis of the knee. A preoperative level of central sensitization is associated with reduced pain alleviation following arthroplasty, even when articular nerve fibers that stimulated central sensitization are removed.
This might imply that preoperative pain desensitization would reduce the risk of experiencing residual postoperative pain. Since end-stage hip osteoarthritis people are nonresponsive to duloxetine therapy, therefore it is advised to conduct screening of every knee arthroplasty candidate for adding duloxetine therapy to usual care. Treatment must be done in a controlled fashion since adverse events are frequently experienced.
The addition of duloxetine to usual care appears to be valuable for end-stage knee osteoarthritis people having neuropathic-like symptoms (at risk of central sensitization).
BMC Musculoskeletal Disorders
Duloxetine in OsteoArthritis (DOA) study: effects of duloxetine on pain and function in end-stage hip and knee OA – a pragmatic enriched randomized controlled trial
T. Blikman et al.
Comments (0)