Categories
Change Password!
Reset Password!


Up to 40% of people globally suffer from allergic rhinitis. Repeated symptoms of nasal blockage, rhinorrhea, itchy nose, and sneezing are the hallmarks of allergic rhinitis.
House dust mite sublingual immunotherapy tablets are safe and effective for symptomatic management of allergic rhinitis and improving quality of life.
Up to 40% of people globally suffer from allergic rhinitis. Repeated symptoms of nasal blockage, rhinorrhea, itchy nose, and sneezing are the hallmarks of allergic rhinitis. It also has a large economic impact in terms of both direct and indirect expenses, lost productivity at work, and impaired quality of life. Pharmacotherapy and allergen avoidance methods have been the standard interventions for its management. Allergen immunotherapy (AIT) has been recognized as a viable therapeutic option for individuals refractory to pharmacotherapy.
The immune system is modulated by AIT and is now regarded as the only therapy with a possibly long-lasting disease modification. Sublingual immunotherapy (SLIT) and Subcutaneous immunotherapy (SCIT) are two conventional ways to give AIT. In a wide array of meta-analyses and randomized controlled trials (RCTs), both have been shown to be effective. Because of its ease of use and high safety profile, SLIT usage has gained popularity. Tablets and drops are included in the SLIT formulations. Due to their easier-to-use, more reliable allergen dosage, and scientific evidence of clinical effectiveness, SLIT tablets have increasingly supplanted SLIT drops in usage.
The two main strains of the house dust mite (HDM, the most common allergen globally) are Dermatophagoides farinae and Dermatophagoides pteronyssinus. In individuals with allergic rhinitis and asthma, HDM has a well-established causative role. Various meta-analyses of double-blind, placebo-controlled studies of SLIT and SCIT in children and adults with allergic asthma and allergic rhinitis have demonstrated the effectiveness of HDM-specific AIT in people with allergic respiratory diseases. It has been demonstrated that HDM-AIT is efficient in raising the quality of life scores, lowering the need for rescue medicine, and alleviating symptoms.
But, there is a great degree of heterogeneity in outcome criteria, AIT formulations, study demographics, and research designs of the clinical trial. In an indirect comparison of HDM-SCIT and HDM-SLIT using network meta-analysis, SCIT was reported to be more beneficial than SLIT drops and tablets in minimizing allergic rhinitis symptoms. In contrast to the well-designed large double-blind, placebo-controlled trials of SLIT tablets, participant numbers in SLIT- and SCIT drops-RCTs were extremely low. The trials on SLIT and SCIT drops are also very inconsistent in terms of allergen formulations, dosages, and outcome estimations.
The European Academy of Allergy and Clinical Immunology (EAACI) recently recommended a consistent, harmonized definition of combined symptom and medication score (CSMS) to use as a key outcome in AIT studies. In several regions of the world, only two HDM-SLIT tablets have been approved: Index of reactivity (IR)-HDM and Standardized quality (SQ)-HDM.
RATIONALE BEHIND RESEARCH
No meta-analysis has previously examined the effectiveness of SLIT tablets in HDM-induced allergic rhinitis patients utilizing the CSMS as a major endpoint. Therefore this systematic review and meta-analysis was performed.
OBJECTIVE
The current study sought to assess the effectiveness of SLIT tablets on conjunctivitis scores, rhinitis symptoms, medication scores, safety, and quality of life of patients with HDM-induced allergic rhinitis.
Literature search
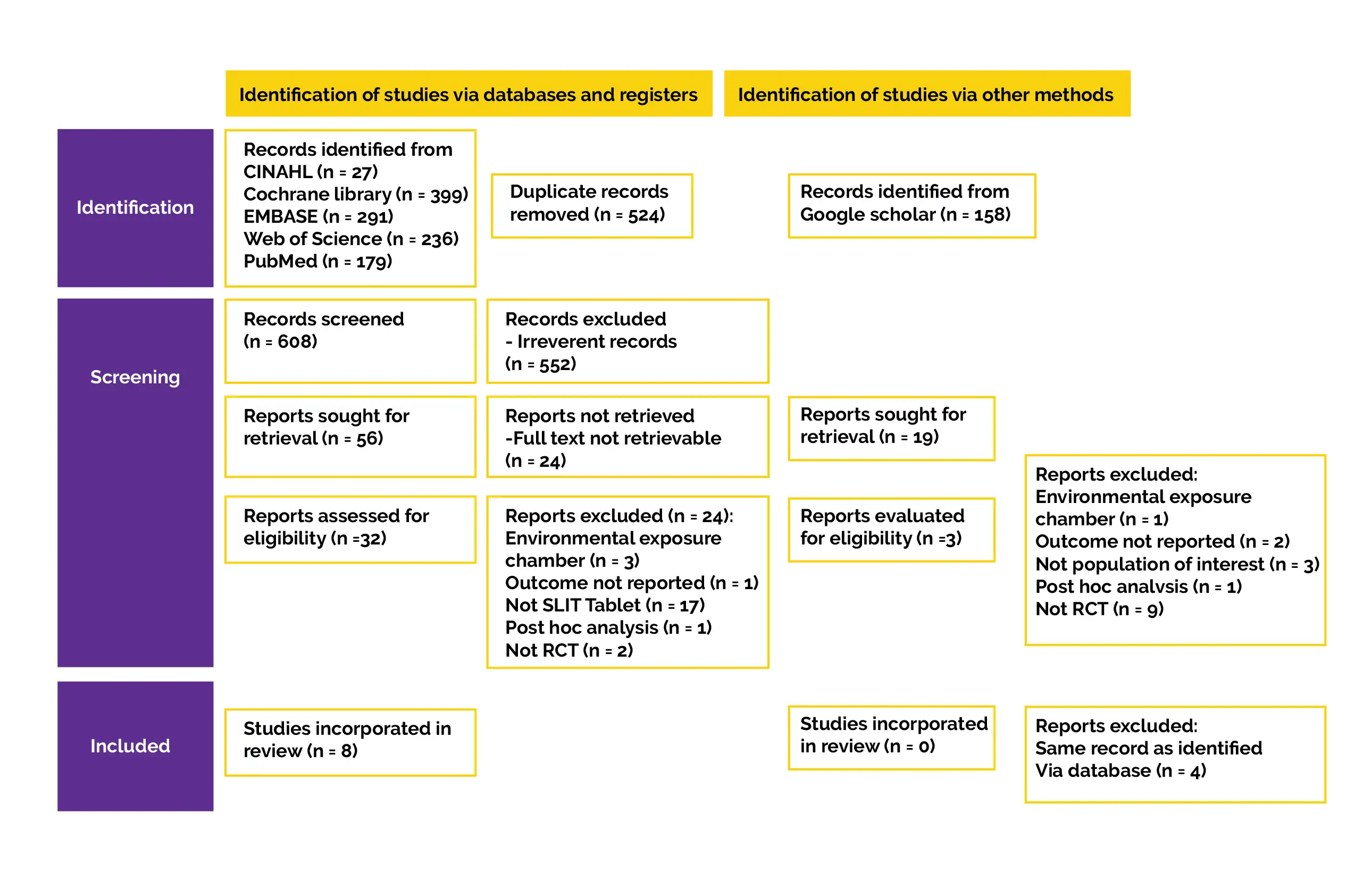
For relevant literature, searches were conducted in five electronic databases, including CINAHL, Cochrane Central Register of Controlled Trials, Web of Sciences, EMBASE, and PubMed. A structured search approach was used to conduct the literature search. Controlled vocabulary terminologies were used particularly for each database, like Medical Subject Heading (MeSH) for PubMed. Grey literature was procured by using Google Scholar's advanced search to find additional studies that were under the control of commercial publishers or not published. Every publication's reference list was checked for any further relevant publications.
Inclusion criteria
Regardless of sample size, RCTs were included if they reported:
Exclusion criteria
The following studies were excluded:
Study selection and Data extraction
Titles and abstracts of all retrieved articles that fulfilled inclusion criteria were screened by 2 authors. The reasons for excluding any study were contrasted and discussed. Any discrepancies were sorted by a consensus meeting with the 3rd author. The key findings, outcome measures, sample size, specifics of the intervention and control, characteristics of patient, inclusion and exclusion criteria, research design, country, year of publication, and authorship were all retrieved separately by the 2 authors.
Data extraction was double-checked, and any differences were reconciled with the third author. The corresponding investigators of studies with insufficient outcome data were contacted via email. If a response wasn't received within two weeks, the data were either recorded as missing or imputed. The accurate mean value with standard deviation (SD) was recovered from full analysis set (FAS), and extraction of participant count was done for continuous outcomes (such as CSMS, Rhinitis Symptom Scores [RSS]).
For studies that only used figures to display the means and SD, the data was retrieved using Digitizelt tool. Wan et al's techniques were used to determine means and SD for studies that reported medians and interquartile range. According to the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions, if SD of the score wasn't provided, imputation of SD was computed utilizing 95% difference in scores between the placebo and HDM-SLIT group. The number of patients who reported feeling better and considerably better at the conclusion of the therapy was extracted for binary outcomes, such as safety or global assessment. The number of volunteers who experienced mild, moderate, or severe treatment-related adverse events (TRAE) in each arm was used to derive the safety result.
Data and Statistical Analysis
Stata statistical package version 17 was used to carry out all statistical analyses. For clinical significance, a 2-tailed, P-value of less than 0.05 was utilized. DerSimonian and Laird's random-effects model was utilized in a meta-analysis to determine pooled estimates and 95% confidence intervals (95% CI). Standardized mean difference (SMD) was used to pool data for continuous outcomes, such as CSMS, which are continuous data with different measurement scales.
Cohen's definition of treatment effects for meaningful interpretation was used to define treatment effects for SMD; a pooled difference of 0.2, 0.5, and 0.8 was regarded as small, medium, and large effects, respectively. For binary endpoints, such as the acceptability of therapy and safety endpoints, pooled relative risk (RR) with 95% CI was used to summarize the data. The I2 statistic, which has a range of 0% to 100% and larger values indicating greater degrees of variability across the research outcomes, was used to measure heterogeneity. I2 values of 75%, 50%, and 25% may symbolize high, moderate, and low heterogeneity, respectively.
Furthermore, a subgroup analysis of subjects receiving 500 IR-HDM, 300 IR-HDM, 12 SQ-HDM or equivalent (20,000 Japanese Allergy Unit [JAU]), or 6 SQ-HDM or equivalent (10,000 JAU) sublingual tablet formulation was conducted. These patients were divided into four groups based on their age (children 5-11 years and ≥12 years old), as well as the dose of treatment. Forest plots were used to display every outcome of the meta-analyses. For ascertaining publication bias, funnel plots were created.
Risk of Bias and Quality assessment
The methodological quality of the incorporated trials was evaluated separately by two researchers, and in the event of a disagreement, a third author was consulted to establish a consensus. The Cochrane group used the Risk-of-Bias 2 (RoB2) algorithm for RCTs to grade the quality of each research.
The following 5 domains comprised the assessment of the methodological quality:
1) Bias brought on by the randomization procedure
2) Bias induced by straying from the desired treatments
3) Bias brought on by the lack of outcome data
4) Bias in estimation of outcomes
5) Selection bias in the reporting of the outcome
Every study received a score using the RoB2 algorithm.
Study outcomes
Outcomes
Study and participant characteristics:
Study quality:
Effect of intervention on the outcome:
In this systematic review of HDM-SLIT tablets, eight placebo-controlled, double-blinded RCTs with adequate information were incorporated. Overall, 3601 patients received HDM-SLIT tablets as treatment, whereas 2783 patients got a placebo. Children, adolescents, and adults who took the HDM-SLIT pill experienced remarkable improvements in their clinical rhinitis symptoms and medicine usage as measured by RMS, CSMS, and RSS. Additionally, it was safe and improved conjunctivitis symptoms and quality of life. This is the 1st meta-analysis to examine CSMS as a major endpoint in accordance with EAACI's suggestion.
The key outcome of the pooled HDM-SLIT tablet exhibited an SMD of -0.28 [95% CI: 0.33 to -0.23]. Both SQ- and IR-HDM SLIT pills demonstrated consistent effectiveness when contrasted to the placebo in a range of doses (6 SQ, 12 SQ, 300 IR, and 500 IR) with minimal variation across trials. The subgroup analysis failed to reveal a difference in the clinical response of RSS and CSMS between each group. A minimal statistically significant impact for AIT was defined by World Allergy Organization (WAO) as > 20% difference in clinical betterment from placebo. Due to lack of data, the pooled difference in clinical improvement could not be determined.
The outcomes were more promising for 12 SQ-HDM tablets (relative difference in TCRS vs. placebo: 22% in 12 SQ-HDM; 18% in 6 SQ-HDM) in a three-arm RCT of 6 SQ-, 12 SQ-HDM tablets, and placebo in 992 adult people suffering from allergic rhinitis. This was true even though there was clinically meaningful betterment across various dosages of HDM-SLIT tablet contrasted to placebo. Twelve SQ-HDM tablets were effective in reducing all secondary outcomes, such as RSS, RMS, combined rhinoconjunctivitis score, and improving quality of life.
For treating allergic rhinitis in adults and adolescents, approval of 12 SQ-HDM tablets may have been influenced by this factor. There were two studies including children under the age of 12 (5 to 18 years of age). These RCTs demonstrated the tolerability and efficacy of the 6 SQ- and 300 IR-HDM tablets in young children. In comparison to adolescents and adults, children may require a lower effective dose of HDM-SLIT. In all the included studies, the HDM-SLIT tablet's lower RMS was less noticeable than the RSS. This might mean that the improvement in the clinical condition is not always adequately correlated with the usage of as-needed pain relief medicine.
Using drug ratings alone to determine the clinical efficacy of AIT might possibly overestimate the true impact of AIT. The European Medicines Agency (EMA) guideline 2008 suggests that assessment of phase-3 AIT trial must be done by natural exposure (field trial). Allergen exposure chamber may be utilized not only for phase 1 and phase 2 or to confirm findings of phase-3 field trial. Therefore, studies that used an allergy provocation test such as an allergen exposure chamber to assess the key endpoint were not included.
Only 3 studies analyzed HDM-SLIT tablet's effects on ocular symptoms by TCCS. This meta-analysis supported a prior systematic review finding that individuals with allergic rhinoconjunctivitis can experience a reduction in both the total and individual ocular symptom ratings with SLIT use. The global evaluation of enhancement for other participants' self-evaluation of intervention effectiveness was considerably better than the placebo group (RR = 1.21 [95% CI: 1.14 to 1.29]). The drop in RQLQ score compared to placebo with SMD of -0.23 [95% CI: 0.29 to -0.16] showed an improvement in quality of life. The effectiveness of HDM-SLIT tablet was verified by all of these pooled data with moderate to low heterogeneity.
Unlike SCIT, no long-term monitoring of data on safety of SLIT tablets is there. It has been illustrated that SLIT may be safer than SCIT. Although SLIT has several documented local adverse events, severe systemic side effects are uncommon. There were no documented fatalities in the clinical trials for the SLIT tablet conducted in the United States. A total of 35 individuals received epinephrine (0.2% of the 8152 SLIT-associated adverse events). None of them was regarded as critical or life-threatening. In accordance with this systemic review, the treatment group's rate of TRAE was much greater than the placebo group's (65.1% vs. 22.7%). The number of severe events was just 0.3%.
During the trials, epinephrine was administered to 8 people from the treatment group (0.20%) where the rate was comparable to the rate for the 7 persons in the placebo group (0.24%). Although systemic SLIT responses are not common, unpleasant local unfavorable side effects are frequently the cause of therapy termination. According to the present meta-analysis, patients who took HDM-SLIT tablets had a substantially increased chance of experiencing TRAE-related cessation (RR 2.09; 95% CI: 1.27 to 3.44).
The clinical trials of HDM-SLIT tablets were only recorded for a one-year treatment period, despite the fact that the current standards call for SLIT treatment to last for at least three years. The persistent advantage of symptom improvement during 2nd year was demonstrated in a 2-year double-blinded RCT on HDM-SLIT tablets with intervention for 1st year and observation without intervention in 2nd year. This can raise the issue of whether a 3-year treatment period is indeed necessary.
Children with allergic rhinitis were managed with grass pollen-SLIT tablets for three years in a well-designed, double-blind large, placebo trial. In children treated with SLIT 2 years following SLIT was stopped, it showed a persistent reduction in allergic rhinitis symptoms, a substantial decrease in asthma symptoms, and medication use. It is uncertain if other allergen-SLIT tablets have the same long-term therapy effectiveness and disease-modifying impact. It is necessary to conduct a longer-term RCT for treatment and monitoring using HDM-SLIT tablets.
In summary, the present systematic review and meta-analysis showed that for easing the symptoms of allergic rhinitis and minimizing the need for medication, HDM-SLIT tablets are an efficient intervention. They also lessen conjunctivitis symptoms and enhance the quality of life. In allergic rhinitis patients older than five years, the efficiency of therapy has been portrayed. Although treatment-related side effects are frequent, the majority are temporary and mild.
Epinephrine was infrequently used in response to serious reactions. Although the HDM-SLIT tablet has been regarded as a safe and efficient therapeutic option, its application in real-world practice is still quite restricted in several nations. The length of therapy, lack of accessibility to therapeutic products, high cost, patients' limited knowledge, and practitioners' lack of expertise with SLIT are all significant hurdles.
In people with allergic rhinitis, HDM-SLIT tablets have a favorable safety profile and their use is associated with effective reduction in rhinitis symptoms, conjunctivitis symptoms, and medication consumption. Furthermore, they also enhance patients' quality of life.
World Allergy Organization Journal
Efficacy and safety of house dust mite sublingual immunotherapy tablets in allergic rhinitis: A systematic review and meta-analysis
Prapasri Kulalert et al.
Comments (0)