Categories
Change Password!
Reset Password!


A double‐blind, placebo‐controlled, multicenter study was conducted to explore the safety, efficacy, and tolerability of AK0529 (oral RSV fusion protein inhibitor) in the treatment of respiratory syncytial virus (RSV) infection, involving both single and multiple oral dosages.
In infants (aged 1–24 months) afflicted by RSV infection, AK0529 (Ziresovir) is well-tolerated and effectively reduces viral load and Wang Respiratory Score.
A double‐blind, placebo‐controlled, multicenter study was conducted to explore the safety, efficacy, and tolerability of AK0529 (oral RSV fusion protein inhibitor) in the treatment of respiratory syncytial virus (RSV) infection, involving both single and multiple oral dosages.
This phase 2, proof‐of‐concept, randomized trial involved infants aged 1–24 months who were hospitalized due to RSV infection. In the first part of the study, 24 volunteers were randomly assigned in a 2:1 ratio to get a single dose of AK0529 up to 4 mg/kg or a placebo. In the second part, 48 subjects were randomized 2:1 to get AK0529 at doses of 0.5, 1, or 2 mg/kg twice daily, or a placebo, over a period of 5 days.
Population pharmacokinetics modelling was used to analyze sparse pharmacokinetic samples. The study evaluated aspects like respiratory signs and symptoms, viral load, tolerability, and safety on a daily basis throughout the intervention duration.
AK0529 exhibited no significant safety or tolerability concerns, as indicated by the occurrence of grade ≥3 treatment-related adverse events in 4.1% of AK0529 subjects and 4.2% of those in the placebo group, with no instances leading to mortality or study withdrawal. In the second part of the trial, the targeted drug exposure was successfully achieved with a dosage of 2 mg/kg twice daily.
Notably, a higher decline in median viral load at 96 hours was observed in the AK0529 group compared to the placebo group. In comparison, the AK0529 group showed a more pronounced decrease in the Wang Respiratory Score from baseline to 96 hours compared to the placebo group (Table 1).
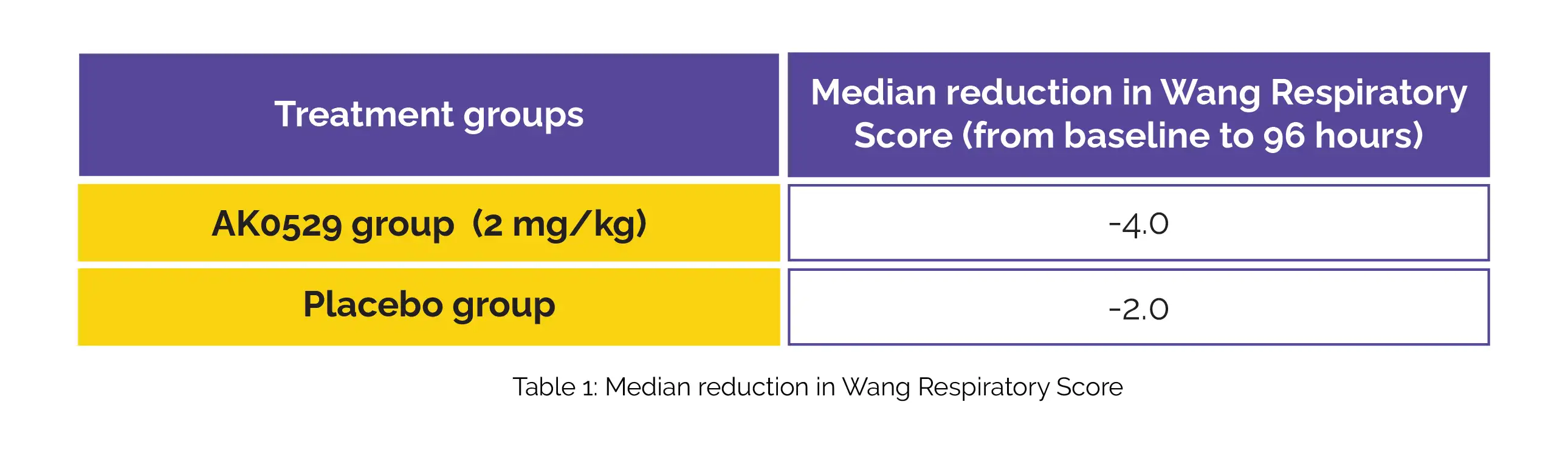
In hospitalized infants suffering from RSV infection, AK0529 exhibited a good tolerability profile. Furthermore, it demonstrated a reduction in viral load and Wang Respiratory Score when administered at a dose of 2 mg/kg twice daily.
Influenza and Other Respiratory Viruses
Safety and efficacy of AK0529 in respiratory syncytial virus-infected infant patients: A phase 2 proof-of-concept trial
Li‐Min Huang et al.
Comments (0)