Categories
Change Password!
Reset Password!


In this double-blind, randomized, placebo-controlled trial, the effectiveness of the potassium channel blocker drug Fampridine was investigated in multiple sclerosis patients with myelitis.
In multiple sclerosis patients with acute phase of cervical transverse myelitis, treatment with Fampridine and intravenous Methylprednisolone reduced illness symptoms and enhanced daily activity capacity.
In this double-blind, randomized, placebo-controlled trial, the effectiveness of the potassium channel blocker drug Fampridine was investigated in multiple sclerosis patients with myelitis.
A total of 30 individuals who experienced their first episode of cervical myelitis with quadriparesis presentation and a final diagnosis of multiple sclerosis were randomly segregated into 2 equal groups. Volunteers in the intervention group got Fampridine together with intravenous Methylprednisolone for 7 days. Intravenous Methylprednisolone was administered for 7 days plus a placebo to the control group. The Barthel index (BI) scores of the groups were examined at the beginning of study and 21st day following the commencement of intervention to compare treatment results.
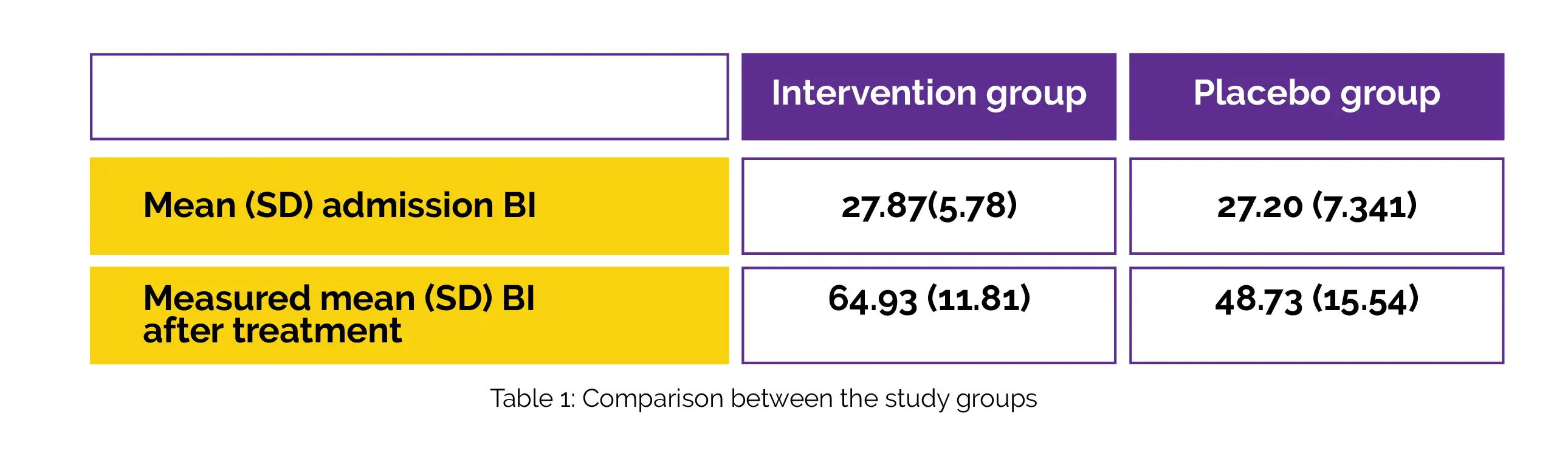
Regarding mean age, sex, and mean admission BI, there was no discernible difference between the intervention and placebo groups at baseline. The mean (Standard deviation [SD]) admission BI, and the measured mean (SD) BI in the intervention group and the placebo group after 3 weeks are depicted in Table 1:
The combination of Fampridine and intravenous Methylprednisolone was beneficial for the management of multiple sclerosis with acute transverse myelitis.
Neurological Sciences
Fampridine in multiple sclerosis patients with acute phase of cervical transverse myelitis: a double-blind, randomized placebo-controlled trial
Ali Amini Harandi et al.
Comments (0)