Categories
Change Password!
Reset Password!


This study aimed to evaluate the effects of oral corticosteroid in Mepolizumab-treated people with severe eosinophilic asthma (SEA).
Prednisolone proved to have limited effects on symptom relief and quality of life but demonstrated favorable impacts on lung function and anti-inflammatory markers in Mepolizumab-treated patients with severe eosinophilic asthma.
This study aimed to evaluate the effects of oral corticosteroid in Mepolizumab-treated people with severe eosinophilic asthma (SEA).
This randomized, triple-blind, placebo-controlled crossover trial comprised adults with SEA. They were subjected to Mepolizumab therapy for a minimum of 12 weeks. The study participants were administered Prednisolone dosed at 0.5 mg/kg/day (maximum 40 mg/day) for 14 ± 2 days in one study arm, and placebo in the other.
The variations in asthma signs and symptoms, quality of life, lung function through spirometry and airway oscillometry, fractional exhaled nitric oxide levels, along with blood and sputum eosinophil cell counts after the Prednisolone and placebo treatments, were assessed.
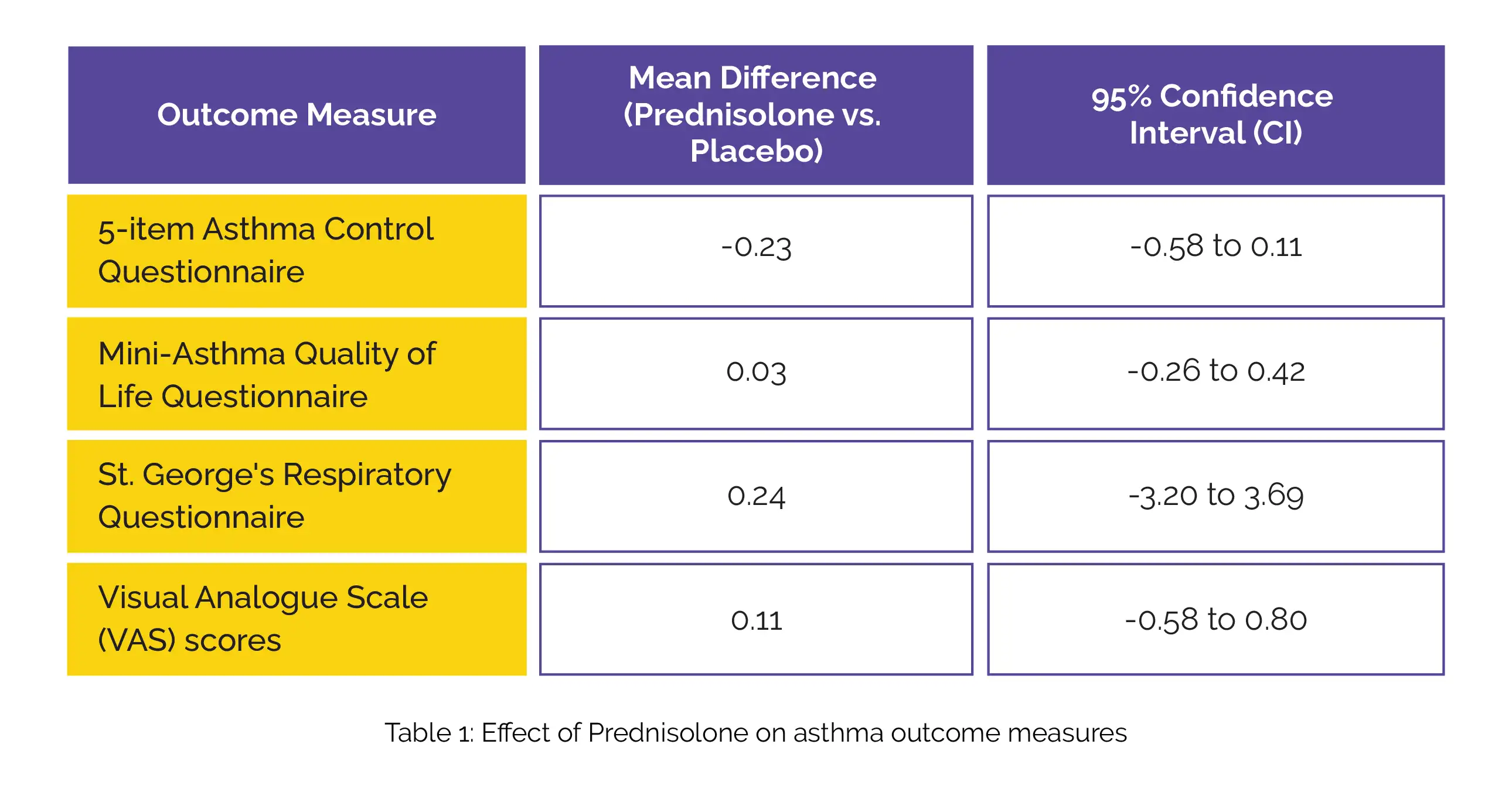
Overall, 27 patients completed the trial. No significant improvement was observed with the use of Prednisolone in these outcome measures (Table 1):
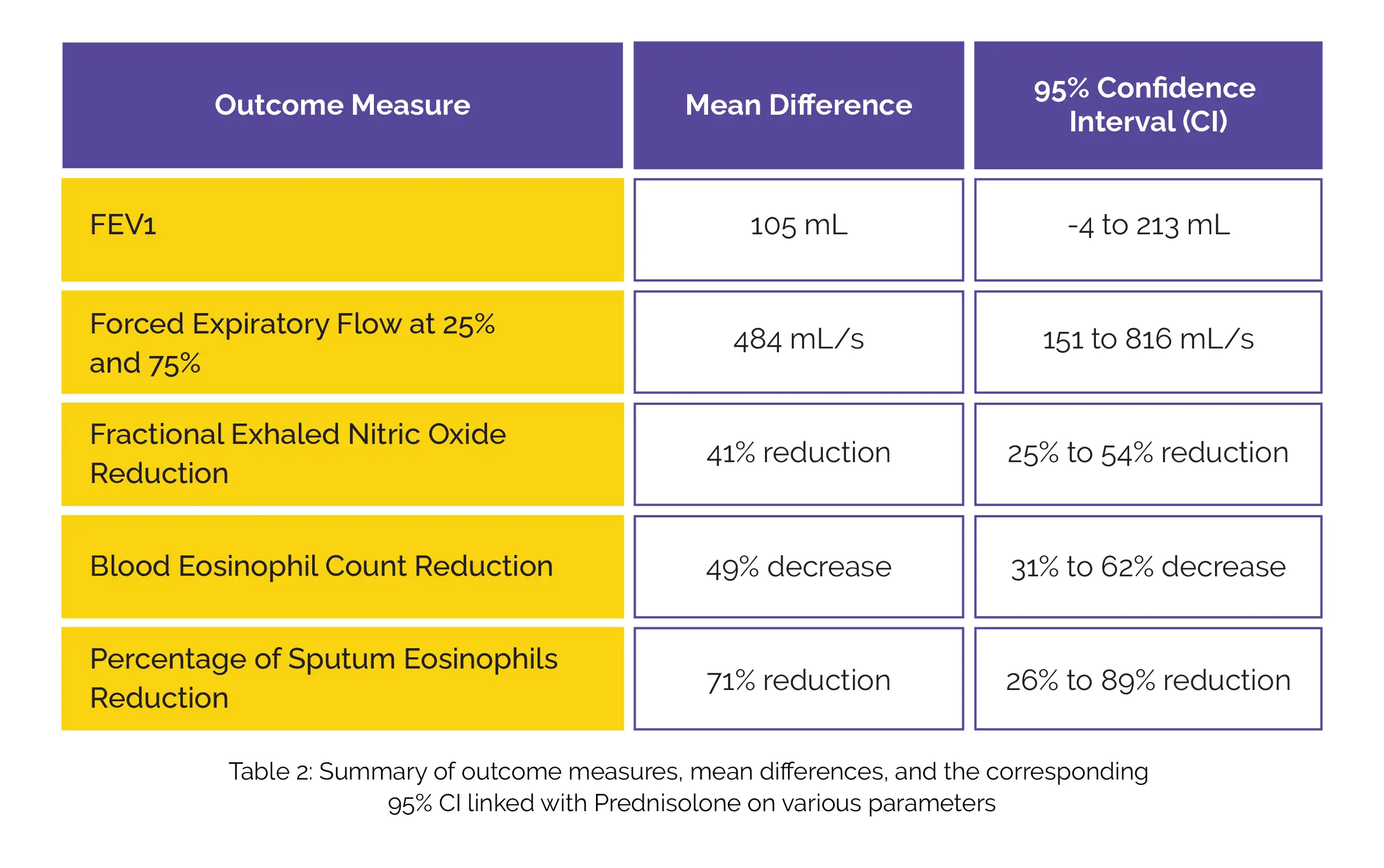
However, Prednisolone did yield a mean difference in favor of forced expiratory volume in 1 second (FEV1) and forced expiratory flow along with a reduction in fractional exhaled nitric oxide, blood eosinophil count, and percentage of sputum eosinophils (Table 2):
While oral corticosteroid improved small-airway obstruction and reduced biomarkers associated with type 2 inflammation, it did not significantly impact symptoms or quality of life in people with SEA who were already receiving Mepolizumab.
The Journal of Allergy and Clinical Immunology
Corticosteroid Responsiveness Following Mepolizumab in Severe Eosinophilic Asthma-A Randomized, Placebo-Controlled Crossover Trial (MAPLE)
Freda Yang et al.
Comments (0)