Categories
Change Password!
Reset Password!


To evaluate the effectiveness of fremanezumab in migraine patients.
This post hoc analysis supported the use of fremanezumab therapy in males and females of 18-70 years suffering from difficult-to-treat migraine. As found, the use of fremanezumab was consistent across various age groups and sex under consideration.
To evaluate the effectiveness of fremanezumab in migraine patients.
The patients who fulfilled the eligibility criteria were randomized to receive quarterly fremanezumab, monthly fremanezumab, or placebo for 12 weeks. The variation from starting in monthly migraine days and other secondary outcomes in pre-defined age (18–45 years and more than 45 years) and sex subgroups were assessed.
A total of 837 patients comprising of 138 male patients; 699 female patients; 373 patients aged 18 to 45 years and 464 patients aged more than 45 years were included. A uniform pattern was observed in decreases in the monthly average number of migraine days during the 12 weeks’ duration irrespective of age (Table 1) and sex (Table 2):
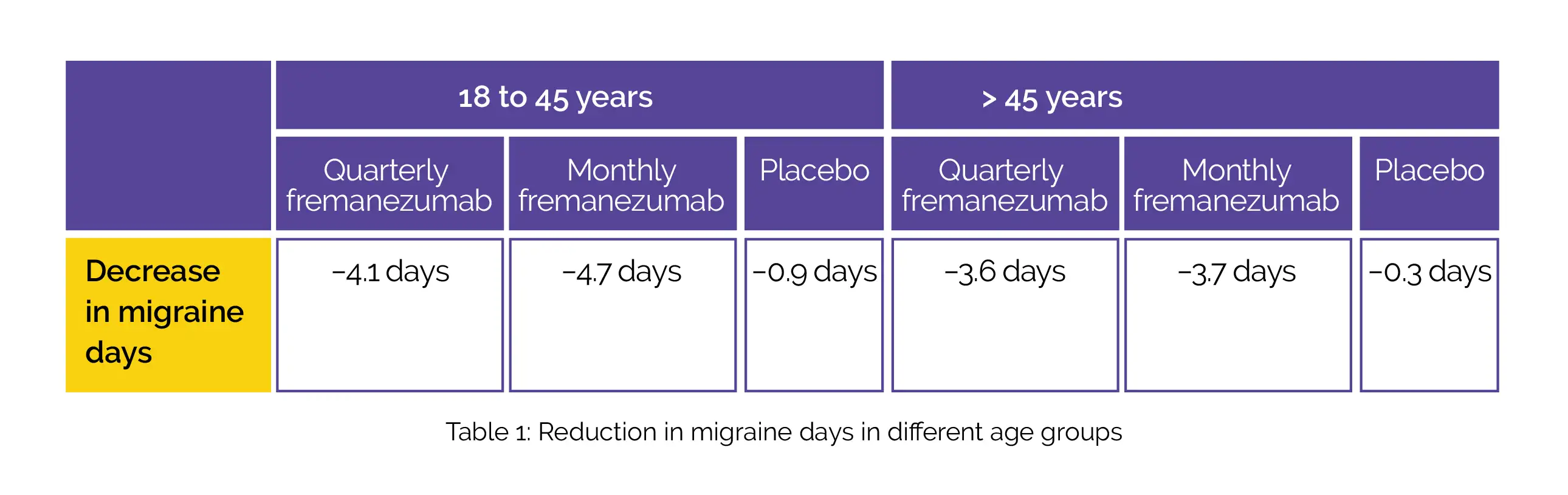
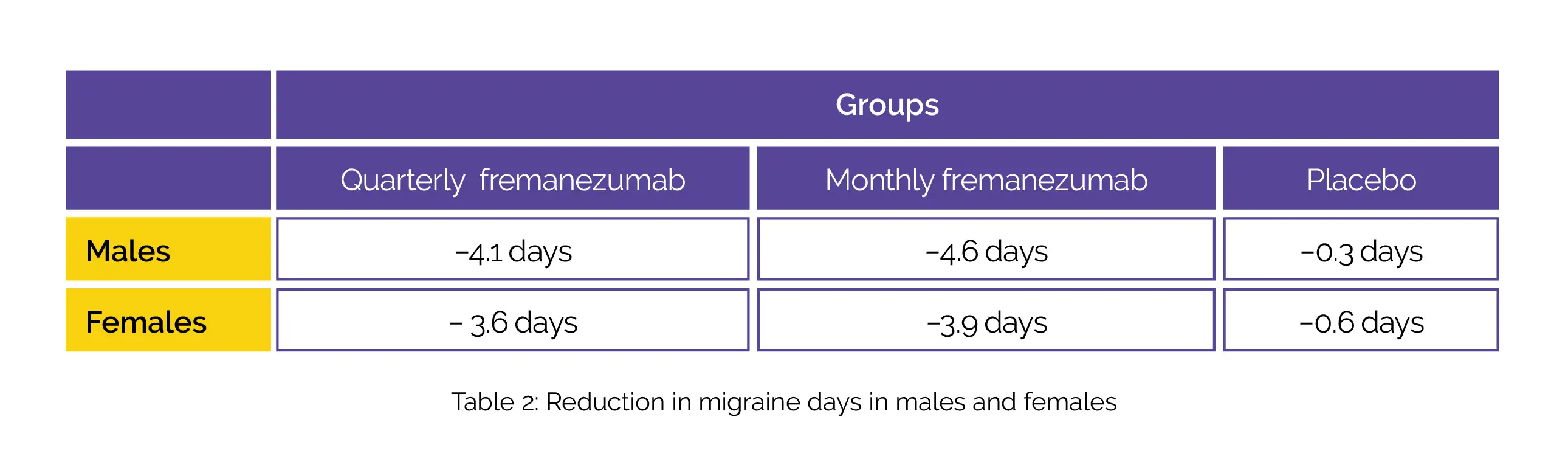
The use of fremanezumab led to a decline in headache days of slightly moderate severity, monthly days of medicine usage, and better Migraine Disability Assessment scores (MIDAS).
Fremanezumab in patients with difficult-to-treat migraine lowered the number of migraine and headache days, extreme medicine use, and disability, irrespective of sex or age.
The Journal of Headache and Pain
Impact of age and sex on the efficacy of fremanezumab in patients with difficult-to-treat migraine: results of the randomized, placebo-controlled, phase 3b FOCUS study
Antoinette MaassenVanDenBrink et al.
Comments (0)