Categories
Change Password!
Reset Password!


Acid peptic disorders (APDs) are a set of conditions manifesting in the form of gastroesophageal reflux disease (GERD), peptic ulcer disease (PUD), dyspepsia and gastritis.
Omeprazole is an effective proton pump inhibitor for treating acid peptic disorders and prevents bleeding risk, with good tolerability, superiority over placebo, and comparable effectiveness to other PPIs for heartburn and ulcer healing.
Acid peptic disorders (APDs) are a set of conditions manifesting in the form of gastroesophageal reflux disease (GERD), peptic ulcer disease (PUD), dyspepsia and gastritis. A global survey of 206 countries in 2019 reported 309.38 million APDs.
GERD: It is characterized by gastric acid reflux into the esophagus causing a sensation of heartburn, nausea, chest pain and chronic cough. The prevalence of GERD has been reported to be 7% to 52% worldwide and from 8% to 30% in India. GERD is further characterized into 3 categories i.e.,
Non-erosive reflux disease (NERD): More common, Prevalence rate; ~70%
Erosive esophagitis (EO): Prevalence rate; 10-30%
Barrett’s esophagitis (BE): It is a result of intestinal metaplasia of the esophagus. A systematic review confirmed the prevalence of endoscopic BE to be 7.8% and histologically confirmed BE to be 1.3 % using clinical trial data across 41 Asian countries.
PUD: It is caused by Helicobacter pylori infection or is NSAID induced. This condition is characterized by the development of ulcers in the lining of the stomach and esophagus. Dyspepsia: It is characterized by chronic pain in epigastric region. A comprehensive study across 40 countries from the year 1990-2022 estimated the global pooled prevalence of dyspepsia to be 8.4%.
Gastritis: It manifests in the form of inflammatory lesions in the mucosal lining of the stomach and can be classified as erosive and non-erosive.
Suggested Treatments for APDs:
Omeprazole: Drug of choice for various gastrointestinal disorders:
Omeprazole, with a documented safety record of over 30 years, is approved for treating acid-related conditions and effectively manages dyspepsia, as well as healing and preventing NSAID-associated ulcers in the stomach and duodenum.
Omeprazole, a proton pump inhibitor, is a weak base with a pKa of 4.0. After being absorbed in the intestine, it travels through the bloodstream and reaches the parietal cells of the stomach. Omeprazole is not charged at a pH of 7 and can cross cell membranes. However, in the secretory canaliculus of actively secreting gastric parietal cells, where the drug is exposed to a pH of less than 2, Omeprazole becomes protonated and is converted to the active form, a sulfenamide that reacts covalently with the sulfhydryl groups of cysteine residues on the extracellular surface of the alpha subunit of the H(+)/K(+)-ATPase and inhibits the activity of the enzyme (Figure 1B).
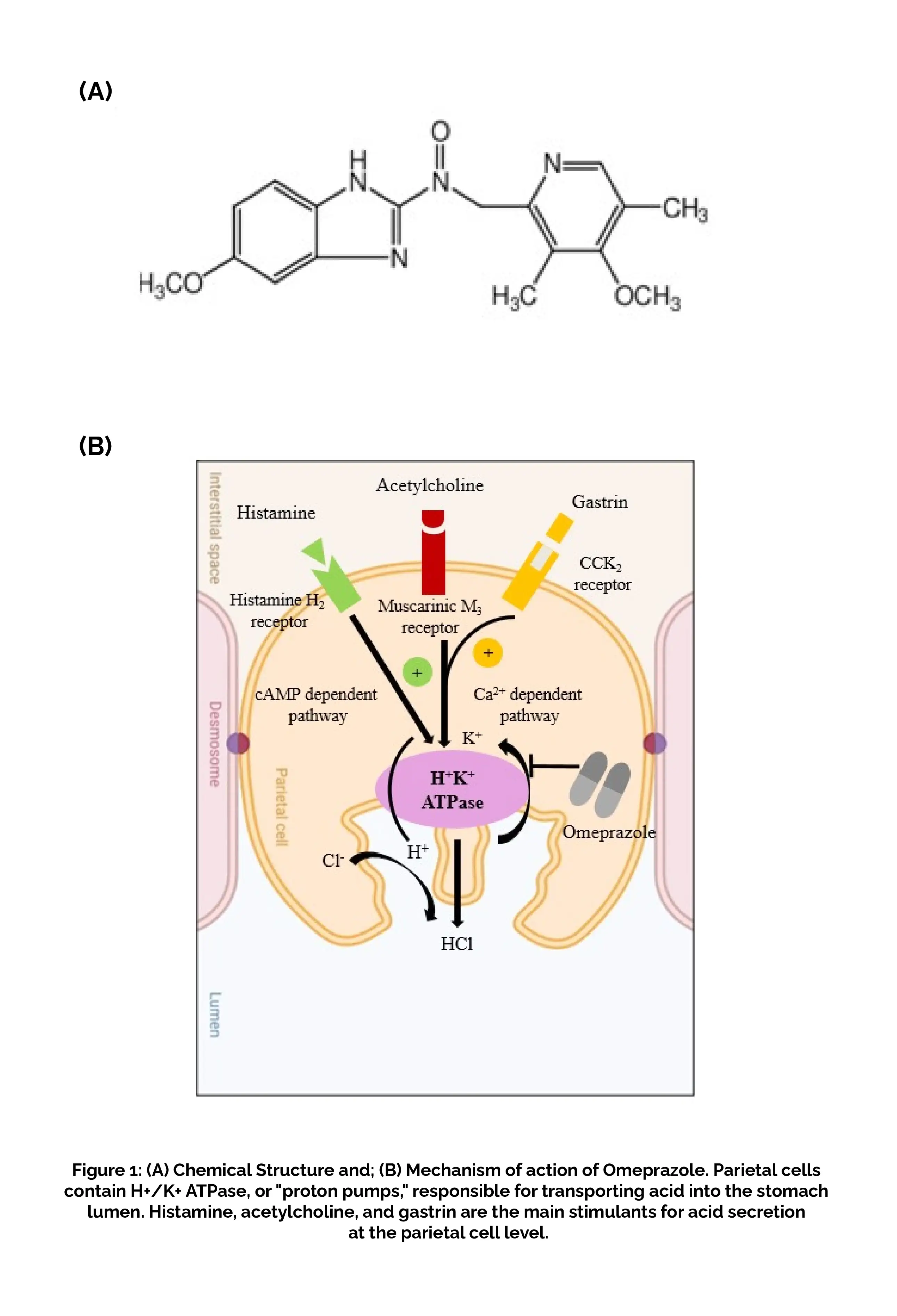
RATIONALE BEHIND RESEARCH
There is a large gap in the determination of the most effective and safe PPI. Furthermore, nothing is known about the comparative evaluation of PPIs for various APD disorders.
OBJECTIVE
This study aimed to investigate the effectiveness and safety of Omeprazole with respect to other PPIs for different types of APDs, such as NERD, EO, etc. based on the different randomized controlled trials (RCTs).
Literature search
To identify prospective RCTs comparing Omeprazole to other PPIs or placebo, PubMed, Google Scholar and the China National Knowledge Infrastructure (CNKI) databases were searched from database inception to 30th March 2023. A combination of specific keywords; ‘GERD’ AND ‘Omeprazole’ AND ‘randomized controlled trial OR cross-over' AND ‘efficacy’ AND NOT ‘prokinetics’ OR NOT ‘alginate’ was used as the search strategy for PubMed. Additionally, reference lists, authors, reviews, meeting abstract websites and https:// clinicaltrials.gov for unpublished trials were used as grey literature resources. The translation and review of articles in languages other than English was also conducted.
Inclusion criteria
This systematic review (following the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses ([PRISMA 2020] guidelines) encompassed the following studies:
Exclusion criteria
Study Selection and Data extraction
The study titles and abstracts were separately evaluated by two reviewers, LV and PM. Data from all full-text articles data were extracted and reviewed individually using a predesigned data extraction form, following standard systematic review and meta-analysis methods.
PICO data was extracted which included:
Data and Statistical Analysis
To be included in the meta-analysis, studies required at least two RCTs or cross-over trials with a common outcome measure by control type (placebo or the same type of PPI). Stata software version 16 was used to generate forest plots and conduct statistical analysis. For significant heterogeneity levels (I2 ≥ 50%), meta-analysis was conducted using Bayesian random effects models, otherwise fixed-effect models were used.
Dichotomous outcomes were assessed using relative risks (RRs) with 95% confidence intervals (CI), and continuous outcomes using standardized mean differences (SMD) with 95% CI. Outcomes were categorized by APD type: NERD, EO, or ulcers (significance threshold of p ≤ 0.05). Heterogeneity among trials was assessed with the I² statistic.
Data on study design, quality, setting, and H. pylori status were collected to identify potential confounders. For any lacking information, the authors were contacted. Assessment of publication bias was performed using funnel plots and Egger's test. The Cochrane Q test was used for subgroup analysis to pinpoint sources of heterogeneity. Sequential sensitivity analysis showed no outcome dependence on any single trial.
Risk of Bias and Quality assessment
The review of each RCT involved independent scoring by coauthors using standard methods. The risk of bias (RoB) was assessed using the RoB 2.0 tool and graded as low, high or some concerns for five types of bias: randomization process; deviations from intended interventions; missing outcome data; measurement of outcome; and selection of reported result.
Study outcomes
Primary Outcomes
Secondary Outcome
Information was gathered about length of hospital stay, cost-effectiveness, nocturnal acid breakthrough, and safety (adverse events).
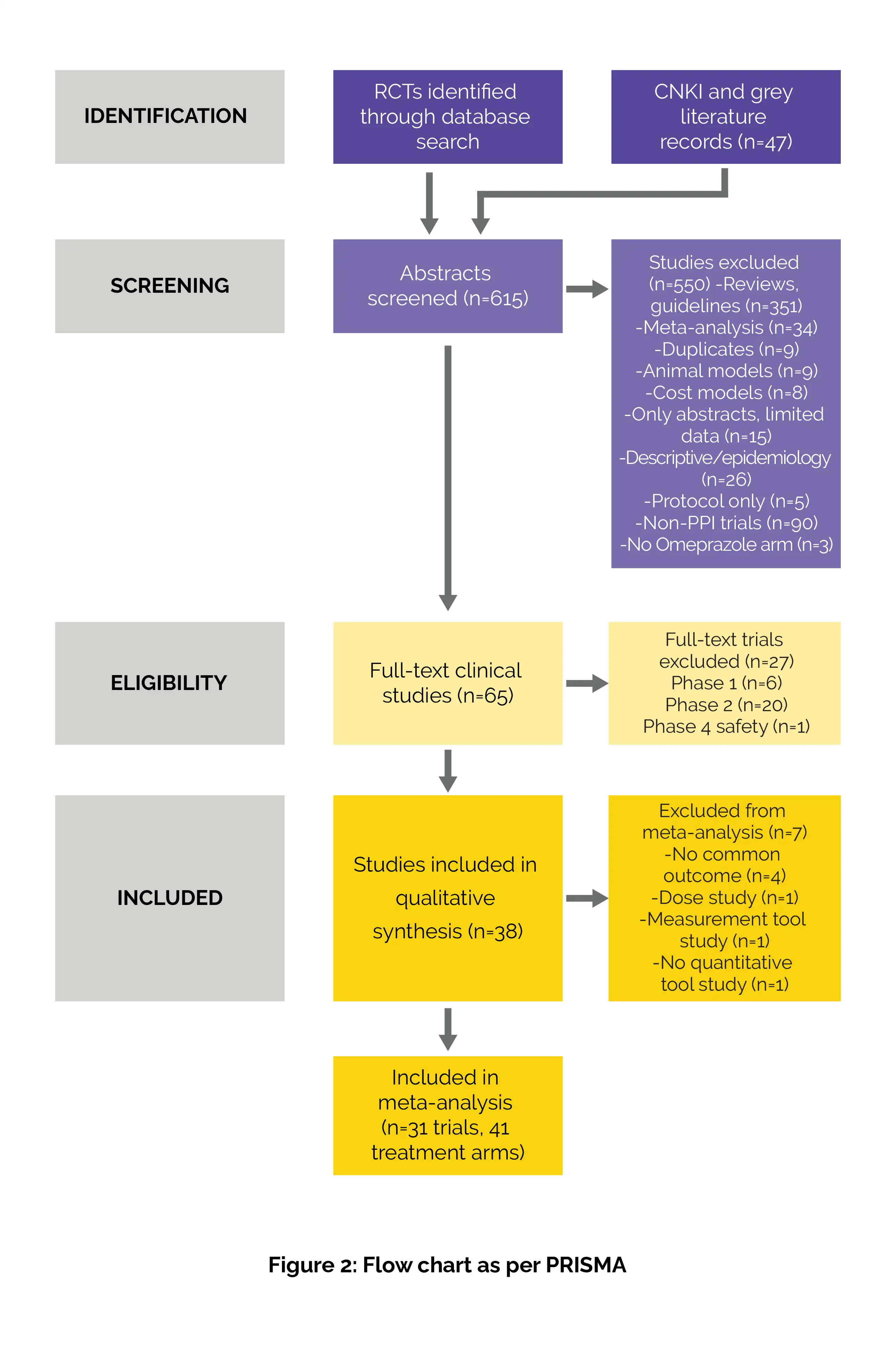
Outcomes
Study and participant characteristics:
Study quality:
Each RCT was independently reviewed and scored for quality and risk of bias using the RoB 2.0 tool. Disagreements were resolved by discussion or a third reviewer. A summary table was created, and study quality was assessed in low-risk trials.
Out of the 31 randomized trials, 68% had low risk of bias and 32% had high risk of bias.
Effect of intervention on the outcome:
This systematic review highlights the role of Omeprazole as an effective treatment for curing the symptoms caused by APDs. This meta-analysis incorporated extensive literature search of articles previously not included due to translation or journal access issues. Moreover, unlike other meta-analysis which studied the effect on only one type of APD, this study incorporates a comprehensive approach towards separate analysis of all PPIs on different types of APDs.
It was discovered that Omeprazole proved to be significantly effective for heartburn relief and displayed signs of overall improvement as compared to placebo. Its efficacy was found to be equivalent to other types of PPIs. Omeprazole was found to outperform lansoprazole in resolving ulcers. However, Edwards et al. reported that the efficacy of esomeprazole in ulcer healing and heartburn relief was significantly better than its racemate; Omeprazole.
A network meta-analysis (62 RCTs) conducted by Dean et al. concluded that PPIs were superior to H2RAs or placebo in healing duodenal ulcers. Another network meta-analysis (23 RCTs) conducted by Barberio et al. confirmed that Omeprazole was effective over esomeprazole in healing the symptoms of NERD.
It has also been established that Omeprazole (20mg/day) provided quick relief from the symptoms in long-term NSAIDs users. All the PPIs, including Omeprazole, have also been found efficient in preventing bleeding caused by NSAIDs. Omeprazole proved to be the most cost-effective of all PPIs in India (89.6 rupees/patient) as well as in a study of Chinese patients with duodenal ulcers (USD $5.30/patient).
Omeprazole had an extremely low chance of side effects (well-tolerable). Merely 11% of the participants reported minor symptoms such as headaches or diarrhoea. Omeprazole has been extensively validated in 1200 clinical trials and 400 million patient treatment courses worldwide. It is a clinically approved medication for the treatment of dyspepsia and gastro-esophageal ulcers with a very well documented long-term safety profile for over 30 years.
Omeprazole's efficacy and cost-effectiveness in treating acid peptic disorders, including GERD symptoms and ulcer healing, underscore its importance as a preferred treatment option, particularly in regions where cost considerations are significant.
International Journal of Clinical Practice
Efficacy and Safety of Omeprazole for the Treatment of Acid Peptic Disorders: A Systematic Review and Meta-Analysis
Mohan Prasad VG et al.
Comments (0)