Categories
Change Password!
Reset Password!


Hepatic fibrosis can advance to cirrhosis of the liver and liver failure if left untreated. This open-label, three-period, multiple-dosage, self-controlled clinical trial was performed to evaluate the pharmacokinetic interaction of Hydronidone and Entecavir in 12 healthy Chinese participants.
Hydronidone is a novel anti-fibrosis drug aimed at reducing hepatoxicity. The use of Entecavir and Hydronidone has been found to be linked with no drug-drug pharmacokinetic interactions in healthy Chinese men.
Hepatic fibrosis can advance to cirrhosis of the liver and liver failure if left untreated. This open-label, three-period, multiple-dosage, self-controlled clinical trial was performed to evaluate the pharmacokinetic interaction of Hydronidone and Entecavir in 12 healthy Chinese participants.
The participants were healthy men from China. They were given Hydronidone (anti-fibrosis agents) 60 mg every 8 hours, for 7 days in phase 1 and then, Entecavir 0.5 mg once daily for 9 days in period 2. After that, Hydronidone and Entecavir combination was given for 6 days (i.e. days 20 to 26). The blood samples were obtained for up to 24 hours after dosing, whereas pre-dose samples were taken on days 7, 19, and 26.
The pharmacokinetic variables of Hydronidone and Entecavir were reported to be similar between combination therapy and monotherapy. But, there was a slight raise in area under the curve (AUC)0–t_ss of Entecavir when used in combination with Hydronidone (from 15.56 ± 2.67 to 16.17 ± 2.77 ng h/ml).
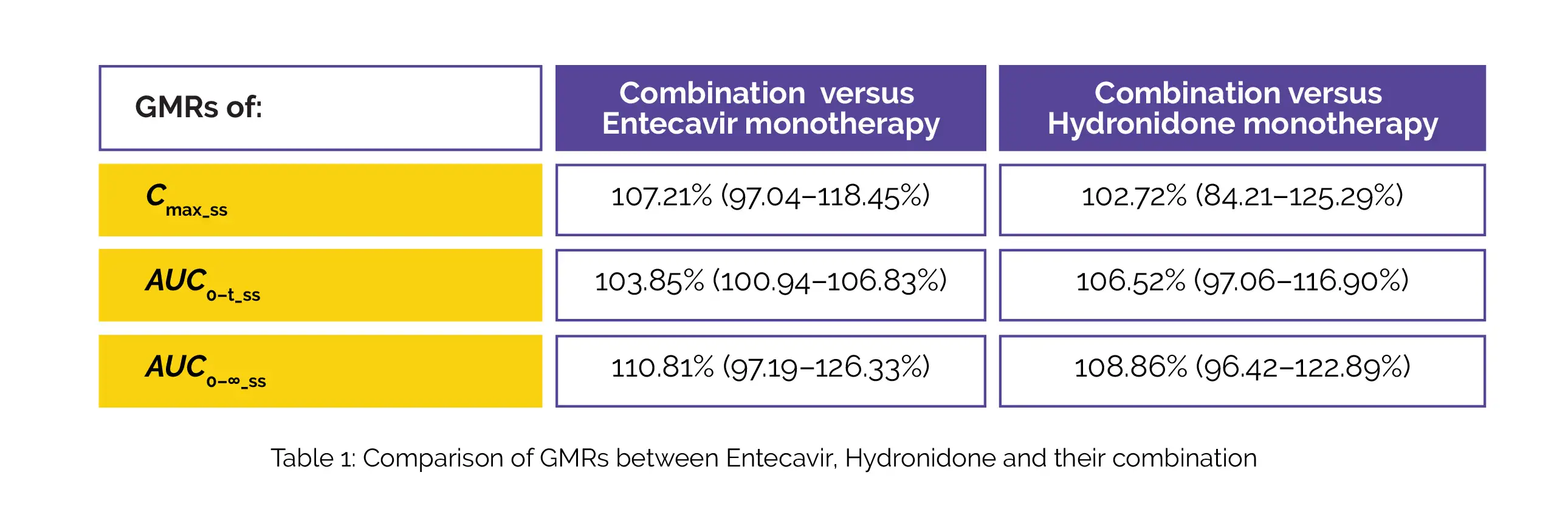
The geometric mean ratios (GMRs) [90% confidence intervals (CIs)] of Cmax_ss, AUC0–t_ss, and AUC0–∞_ss of Hydronidone, Entecavir, and their combination are displayed in below Table 1:
In healthy Chinese men, no drug-drug interaction between Entecavir and Hydronidone was reported. As Hydronidone's multiple doses exhibit a risk with escalating exposure to Entecavir in vivo, future research is required.
Advances in Therapy
A Pharmacokinetic Drug–Drug Interactions Study between Entecavir and Hydronidone, a Potential Novel Antifibrotic Small Molecule, in Healthy Male Volunteers
Rui Zhang et al.
Comments (0)