Categories
Change Password!
Reset Password!


This study was conducted to compare the hospitalization rates in people with moderate to severe atopic dermatitis (AD) treated with dupilumab versus control.
In people with moderate to severe atopic dermatitis, treatment with dupilumab was associated with a substantial reduction in the rate of hospitalization.
This study was conducted to compare the hospitalization rates in people with moderate to severe atopic dermatitis (AD) treated with dupilumab versus control.
In this post hoc analysis, a total of 7 randomized, placebo-controlled trials on subjects with atopic dermatitis who were administered with 300 mg dupilumab every 2 weeks (q2w) or weekly (qw); with or without topical corticosteroids, versus placebo for 12, 16 or 52 weeks were evaluated. Overall, 2932 subjects were enrolled and divided into 2 treatment groups: Dupilumab group and Control group including 1841 and 1091 patients respectively. The endpoints studied were hospitalization rates (“all-cause” and “AD-related”) and length of AD-related hospitalizations.
The rates of all-cause hospitalizations, AD-related hospitalizations and overall duration of AD-related hospitalization are shown in Table 1:
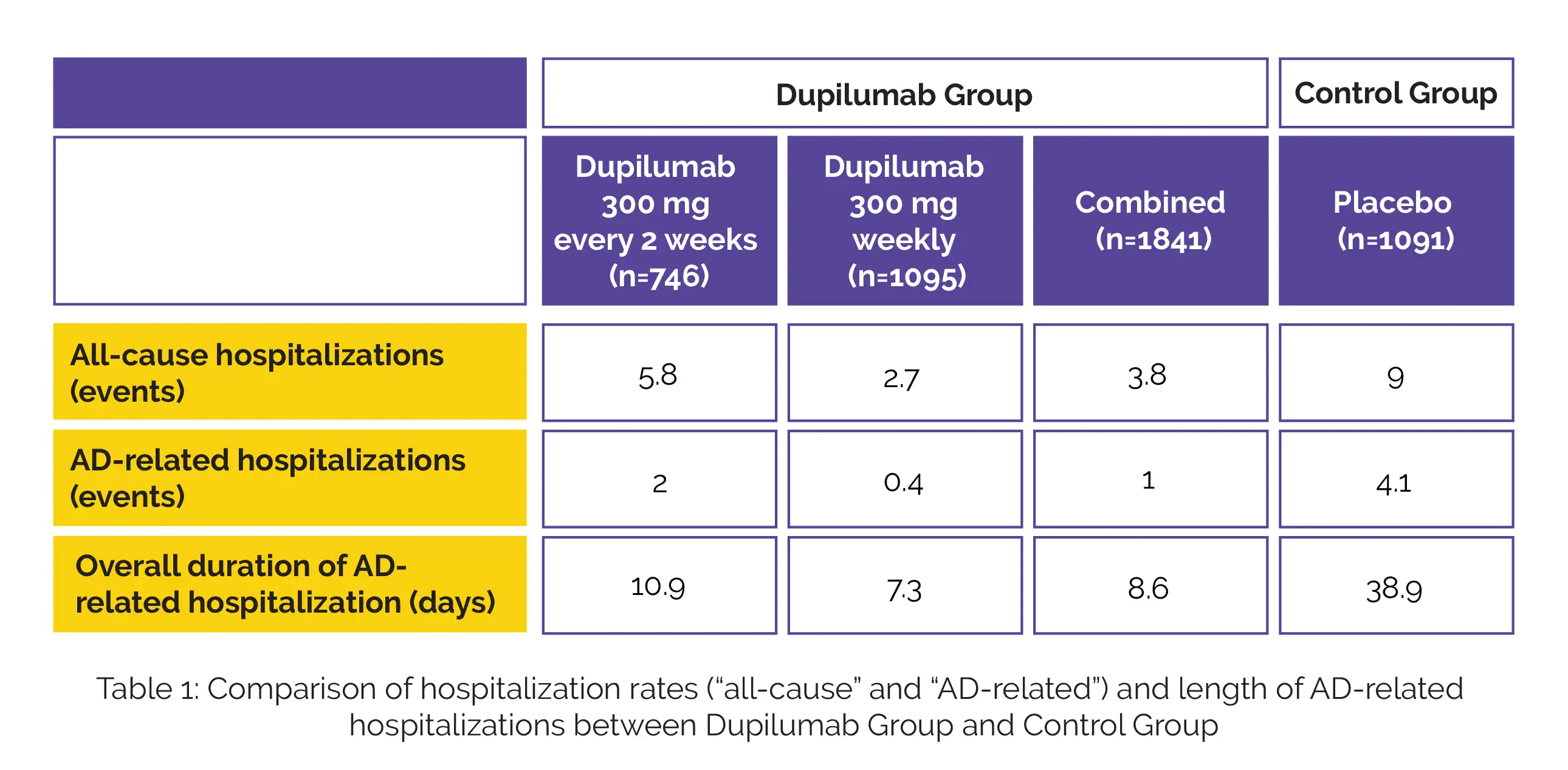
The above findings suggest that moderate-to-severe AD patients who were given dupilumab showed reduced rates of all-cause hospitalizations and shorter hospitalization duration when compared to placebo.
The Journal of Allergy and Clinical Immunology: In Practice
Dupilumab treatment reduces hospitalizations in adults with moderate-to-severe atopic dermatitis
Jonathan I.Silverberg et al.
Comments (0)