Categories
Change Password!
Reset Password!


A post hoc analysis of the PROMISE-2 trial assessed consistency and predictive power of first month eptinezumab therapeutic response (defined by decrease in the frequency of monthly migraine days) on later response outcomes in individuals suffering from migraine.
In people having chronic migraine, early response to the monoclonal antibody eptinezumab illustrates a high likelihood of sustained response, with many people experiencing persistent benefits during the subsequent months.
A post hoc analysis of the PROMISE-2 trial assessed consistency and predictive power of first month eptinezumab therapeutic response (defined by decrease in the frequency of monthly migraine days) on later response outcomes in individuals suffering from migraine.
The PROMISE-2 study randomized chronic migraine people to placebo, 100 mg eptinezumab or 300 mg eptinezumab given intravenously every twelve weeks for up to 24 weeks (two infusions over six study months). Calculation of migraine responder rates (MRRs) was done from monthly migraine days over four-week intervals in comparison with the baseline.
People were grouped by MRR during month 1 (< 25%, 25-< 50%, 50-< 75%, and ≥ 75%), with the number of subsequent study months (months two-six) with ≥50% and ≥ 75% MRR estimated in each subgroup. A similar assessment was carried out utilizing Patient Global Impression of Change (PGIC) rating for defining month 1 subgroups (no change/worse, minimally improved, much improved, very much improved ) and rates of much improved or very much improved PGIC during months two-six.
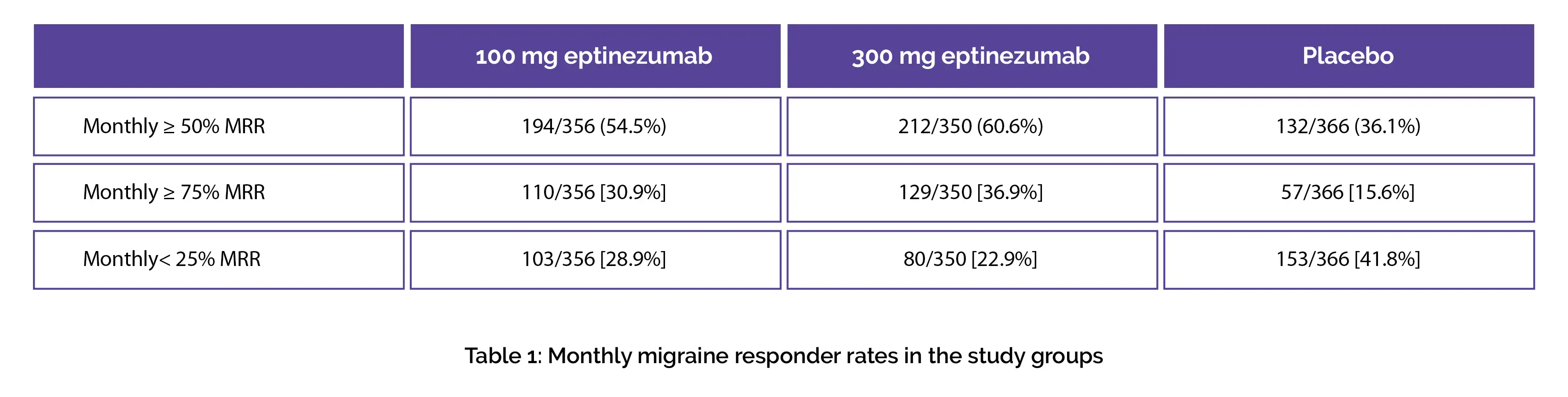
Table 1 shows the percentage of people who were ≥ 50% migraine responders during the first month in eptinezumab 100 mg, 300 mg, and placebo groups. More eptinezumab-treated people were ≥ 75% migraine responders and more placebo-treated people were < 25% migraine responders, as shown in Table 1:
Considering people who attained ≥75% migraine response in the first month, greater than one-third were reported to attain ≥75% migraine response for all the five subsequent study months and greater than two-thirds attained ≥75% migraine response for ≥three months. Notably, greater than two-thirds of those in the very much improved (PGIC) subgroup at the first month reported very much or much improvement for all the five subsequent months.
Hence, more eptinezumab-treated than placebo-treated people were early responders, and majority of the early responders went on to attain a high level of response for at least half of 24-week therapeutic period. The potential for later response in early non-responders was also noted.
The Journal of Headache and Pain
Early response to eptinezumab indicates high likelihood of continued response in patients with chronic migraine
Dawn C Buse et al.
Comments (0)