Categories
Change Password!
Reset Password!


The Reduction of Cardiovascular Events with Icosapent Ethyl Trial (REDUCE-IT) examined if IPE may lessen the extreme cardiovascular disease (CVD) risk in people who smoke cigarettes.
Icosapent ethyl (IPE) therapy was linked with a lower risk of cardiovascular events in existing and former smokers compared to those who never smoked cigarettes. The use of IPE may decrease cardiovascular issues associated with smoking.
The Reduction of Cardiovascular Events with Icosapent Ethyl Trial (REDUCE-IT) examined if IPE may lessen the extreme cardiovascular disease (CVD) risk in people who smoke cigarettes.
This multinational, double-blind, phase 3b trial comprised 8179 patients aged ≥65 years who were on statin therapy and had raised triglycerides and CV risk to IPE or placebo. The median follow-up was 4.9 years. The effect of IPE was assessed in REDUCE-IT as per the smoking history. The groups were categorized as existing smokers (no of people, n = 1241), previous smokers (n = 3672), and never smokers (n = 3264).
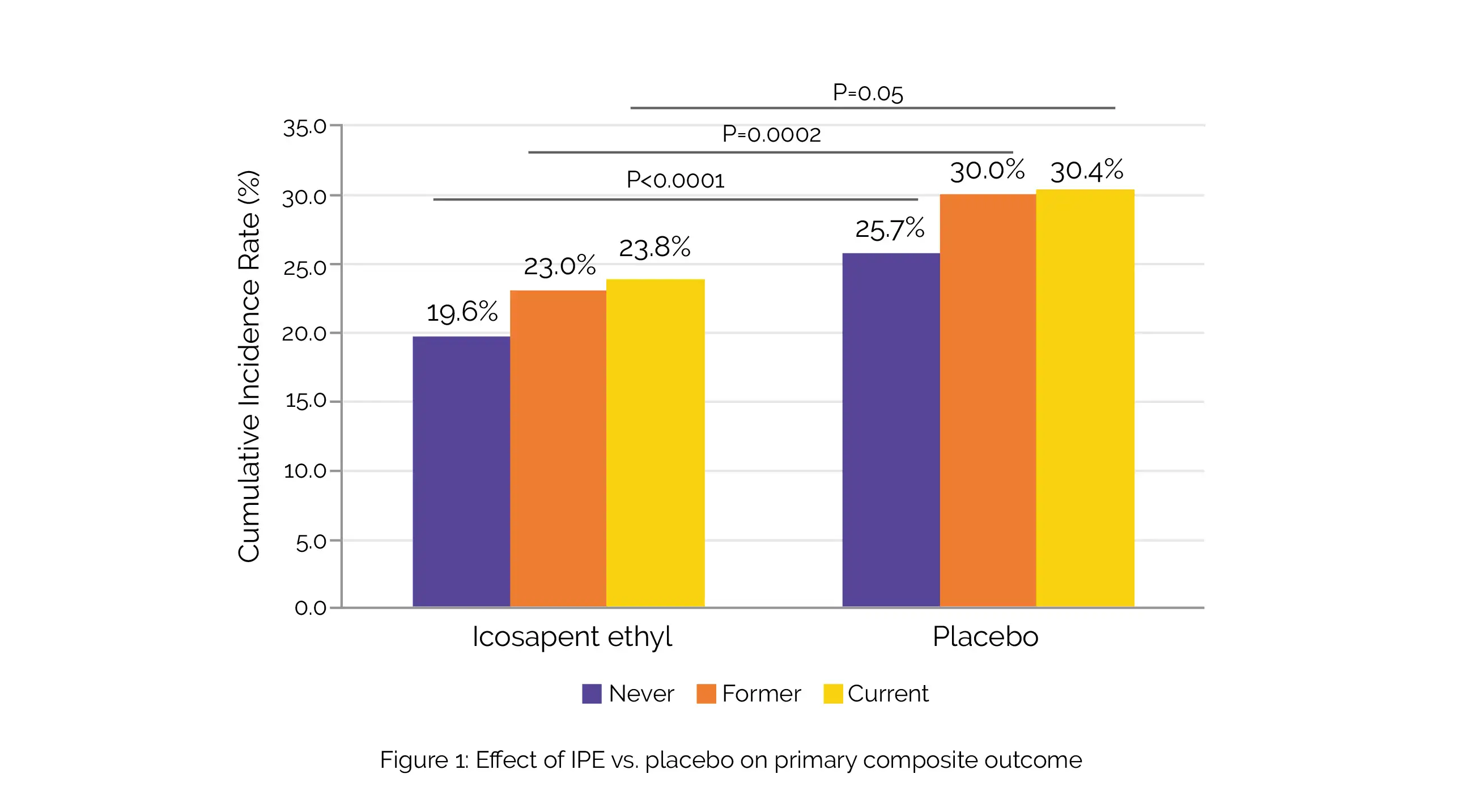
The primary endpoint [death due to CVD, non-fatal myocardial infarction (MI) and stroke, coronary artery revascularization, or hospitalization for unstable angina] lowered by 25% with the use of IPE. Also, IPE therapy was linked with noteworthy reductions in time to the primary composite endpoint (hazard ratio: 0.77) and in overall events (rate ratio: 0.71) in existing plus previous smokers (total number of people, n = 4913).
These advantages remained noteworthy when segmented into existing and previous smokers, with decreases in the secondary endpoint and the specific components of death due to CVD or non-fatal MI and fatal or non-fatal MI, correspondingly. In non-smokers, the advantages were consistent and significant too. Similar expected rates of first incidences of primary CVD endpoints in existing smokers (23.8%) and former smokers (23.0%) allocated to IPE than those who never smoked on placebo (25.7%) were observed (Figure 1).
IPE is a pure form of eicosapentaenoic acid. The outcomes from this analysis raise the likelihood that IPE therapy may decrease cardiovascular issues as a result of smoking. This therapy has been shown to counteract the pro-atherothrombotic effect and incendiary effects of smoking, such as oxidative stress, inflammation, endothelial dysfunction, and platelet hyperreactivity.
European Heart Journal – Cardiovascular Pharmacotherapy
Potential effects of icosapent ethyl on cardiovascular outcomes in cigarette smokers: REDUCE-IT smoking
Michael Miller et al.
Comments (0)