Categories
Change Password!
Reset Password!


This prospective, multicentre, open-label, randomized controlled trial determined if Nebivolol-based hypertension treatment may offer benefits over Telmisartan in minimizing central blood pressure (CBP).
Compared to Telmisartan, Nebivolol-based hypertension therapy may have less potent central blood pressure-lowering effects in patients with hypertension.
This prospective, multicentre, open-label, randomized controlled trial determined if Nebivolol-based hypertension treatment may offer benefits over Telmisartan in minimizing central blood pressure (CBP).
A total of 98 hypertensive patients were enrolled. With a target blood pressure (BP) of ≤140/80, participants were given either Nebivolol-based (49 subjects) or Telmisartan-based (49 subjects) treatment for twelve weeks. The difference in the alteration from baseline central systolic BP (cSBP) following twelve weeks was the key endpoint ascertained.
The baseline central and peripheral SBP of the two groups did not markedly differ from one another. Mean decrease in cSBP from the baseline is shown in Table 1. Peripheral SBP (pSBP) reduced less in the Nebivolol group in comparison with the Telmisartan group.
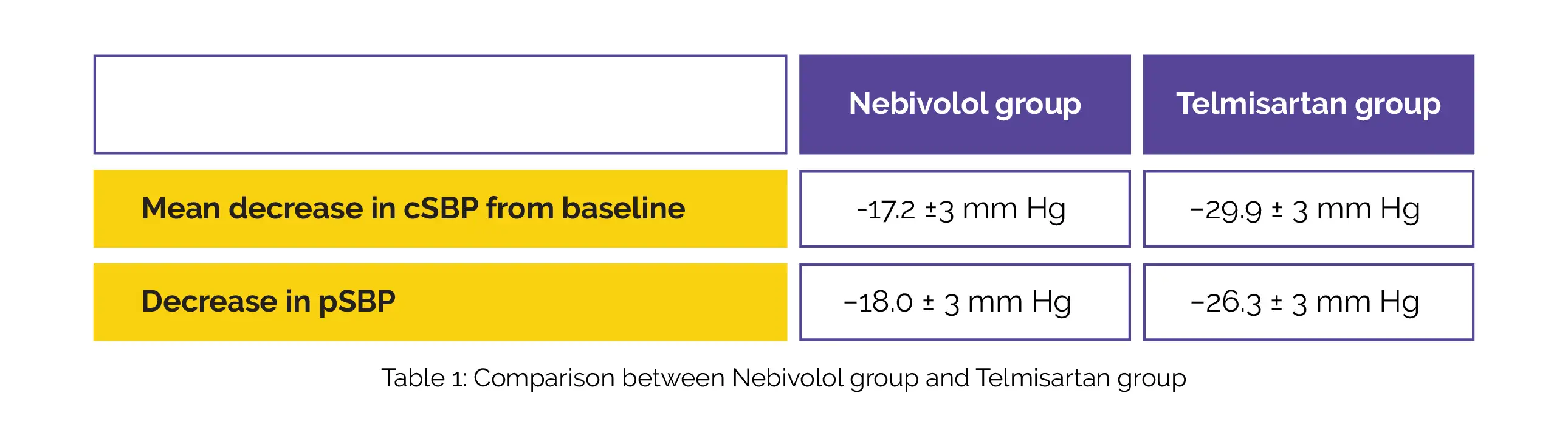
Between the two groups, there was a profound difference in ΔcSBP (12.7 mmHg, 95% confidence interval [CI], 4.13 to 21.2). The ratio of alteration in cSBP and pSBP (ΔcSBP/ΔpSBP; 0.67 for Nebivolol group vs. 1.11 for Telmisartan group) indicates that after controlling for decrease in pSBP, the decrease in cSBP was larger in Telmisartan arm in comparison with Nebivolol arm. But, this difference was not vital.
When opposed to the angiotensin II receptor-blocker Telmisartan, Nebivolol-based hypertension therapy may have less potent benefits in lowering CBP. However, further extensive research is necessary.
European Heart Journal
The differential effects of antihypertensive drugs on central blood pressure: nebivolol versus telmisartan (ATD-CBP)
S Lim et al.
Comments (0)