Categories
Change Password!
Reset Password!


A single-center, open-label trial was conducted to assess the efficacy of cryoneurolysis (cold-induced reversible conduction block of peripheral nerves) for symptomatic relief of ankle osteoarthritis (OA).
In people with unilateral symptomatic ankle osteoarthritis, cryoneurolysis effectively improved ankle pain, function and quality of life for up to 24 weeks.
A single-center, open-label trial was conducted to assess the efficacy of cryoneurolysis (cold-induced reversible conduction block of peripheral nerves) for symptomatic relief of ankle osteoarthritis (OA).
Overall, 40 patients (50% female) aged more than 18 years suffering from radiographic tibiotalar OA, with no ankle surgery within six months of screening, and with unilateral ankle pain ≥5/10 on the Numerical Rating Scale (NRS) were recruited. Evaluation of the outcomes was done by telephone interview (3, 9, 18 weeks) and at clinic visits (6, 12 and 24 weeks) after ultrasound-guided cryoneurolysis of nerves in patient's pain distribution.
Alteration in Foot and Ankle Outcome Score (FAOS) at twelve weeks was the major outcome ascertained. Evaluation of physical performance measures, NRS-pain, activities of daily living (FAOS-ADL), and change in the quality of life (FAOS-QoL) was also done. To assess alterations from baseline at 6, 12 and 24 weeks post-intervention, longitudinal mixed models were constructed.
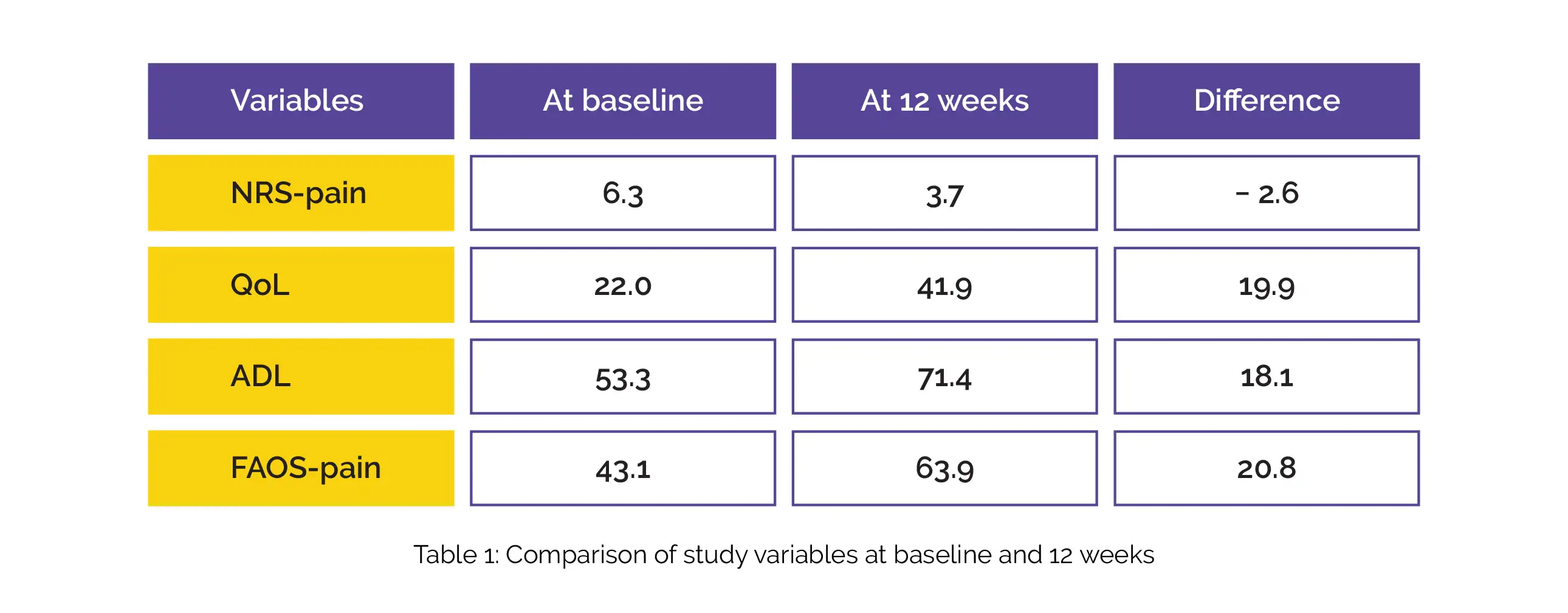
The NRS-pain, QoL, ADL, and FAOS-pain scores were improved from baseline at 12 weeks following treatment, as depicted in Table 1:
At 12- weeks post-treatment, no difference in the 40-m fast-paced walking test was witnessed (-1.2 second). At 6 and 24-week visits, comparable outcomes were seen for all outcomes.
Cryoneurolysis is a safe and efficacious non-pharmacological treatment of joint pain and function in people diagnosed with ankle OA.
Osteoarthritis and Cartilage Open
An open-label, single-arm trial of cryoneurolysis for improvements in pain, activities of daily living and quality of life in patients with symptomatic ankle osteoarthritis
Thomas A. Perry et al.
Comments (0)