Categories
Change Password!
Reset Password!


This systematic review and meta-analysis aimed to evaluate the immunological and clinical effectiveness and safety of local nasal immunotherapy (LNIT) for allergic rhinitis patients.
In people with allergic rhinitis, local nasal immunotherapy is a safe substitute allergen immunotherapy route with no substantial side effects.
This systematic review and meta-analysis aimed to evaluate the immunological and clinical effectiveness and safety of local nasal immunotherapy (LNIT) for allergic rhinitis patients.
Utilizing Embase, Medline, and OVID, trials that compared LNIT and placebo were searched. Nasal provocation threshold, immunological assessment, medication score, symptom-medication score (SMS), and total nasal symptom score (TNSS) were the outcomes ascertained. Pooling of the data was done for meta-analysis. Overall, 20 studies and 698 subjects were incorporated.
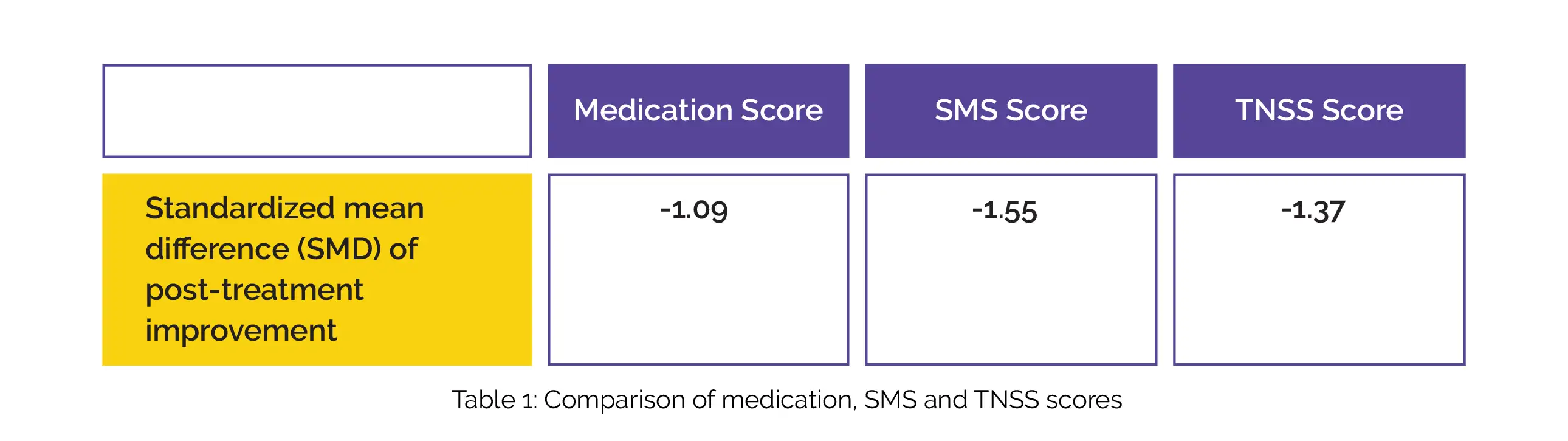
Compared to the control group, the LNIT group had greater post-treatment improvement in medication, SMS and TNSS scores as depicted in Table 1:
No profound differences were witnessed in serum-specific IgE (mean difference [MD] = 6.35), nasal IgE (MD = -0.59), or nasal eosinophil cationic protein (MD= 7.63). Only serum IgG was remarkably elevated with LNIT (MD = 0.45). After the treatment, the nasal provocation threshold was greater with LNIT (MD = 27.30). There were no side effects witnessed.
Local nasal immunotherapy is associated with improvement in clinical symptoms, reduction in medication usage, and elevation in nasal provocation threshold in allergic rhinitis patients.
International Forum of Allergy & Rhinology
Local nasal immunotherapy for allergic rhinitis: A systematic review and meta-analysis
Navarat Kasemsuk et al.
Comments (0)