Categories
Change Password!
Reset Password!


A prospective interventional study was carried out to explore the benefit of adding Lactobacillus reuteri (L. reuteri) to the standard triple therapy to manage Helicobacter pylori (H. pylori) infection.
The addition of Lactobacillus reuteri to the standard triple drug regimen (esomeprazole + clarithromycin + amoxicillin) is associated with higher H. pylori elimination rates.
A prospective interventional study was carried out to explore the benefit of adding Lactobacillus reuteri (L. reuteri) to the standard triple therapy to manage Helicobacter pylori (H. pylori) infection.
The study enrolled 90 people diagnosed with H. pylori infection. Participants were divided into the following groups: (i) Group-I (n=45): Received standard triple drug therapy (Esomeprazole + Clarithromycin + Amoxicillin) for fourteen days, and (ii) Group-II (n=45): Received the combination of standard triple therapy and L. reuteri for fourteen days.
With the aid of the 13C-urea breath test, the H. pylori status was assessed. Using a validated Gastrointestinal Symptoms Rating Scale (GSRS) questionnaire, recording of the symptoms and symptom severity prior to and after the intervention was done.
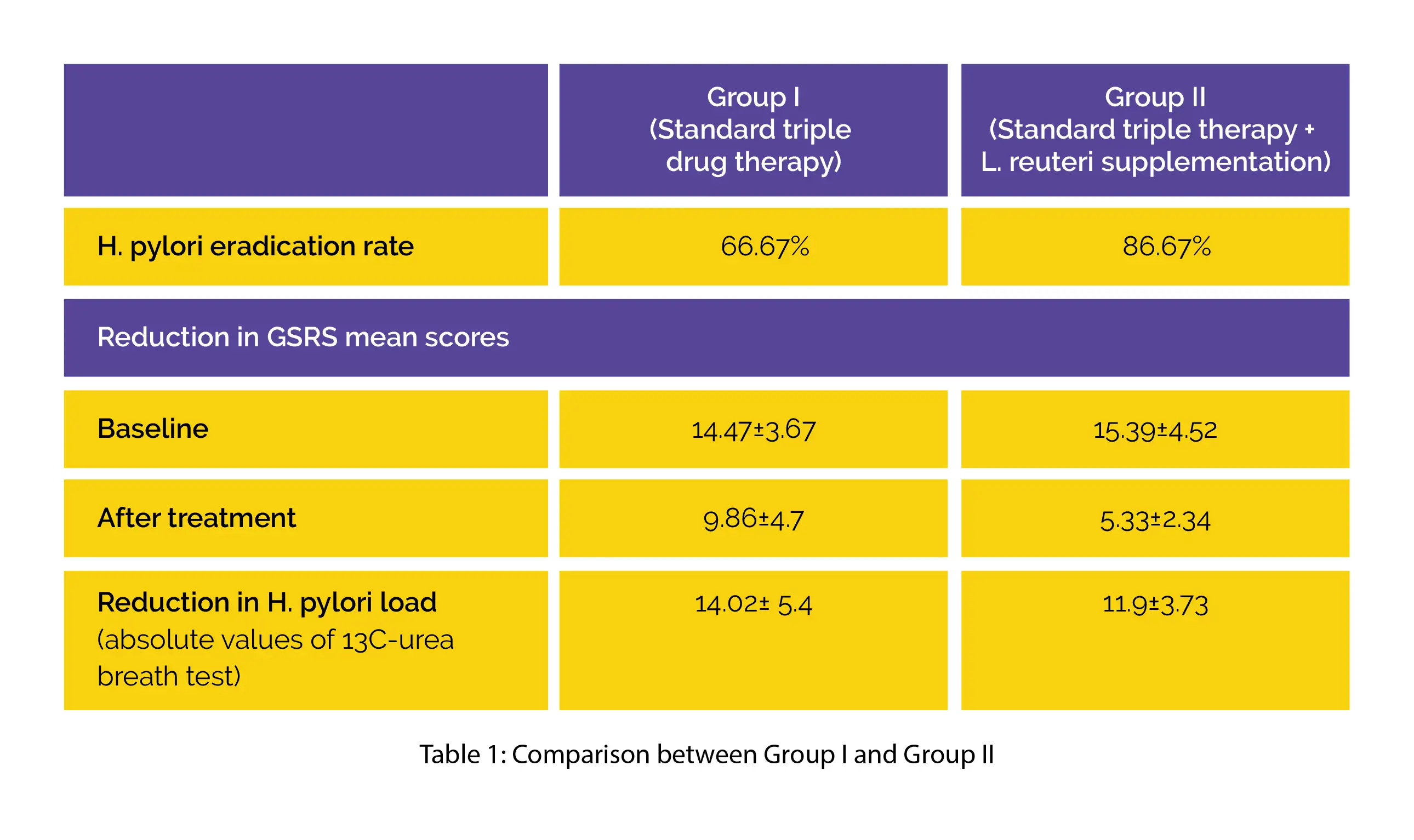
Compared to Group I participants, Group II participants exhibited a remarkably higher H. pylori elimination rate and greater decline in GSRS mean scores. The absolute values of the 13C-urea breath test indicated a greater drop in H. pylori load in Group II when compared to Group I, as illustrated in Table 1:
L. reuteri addition was reported to minimize adverse effects linked with triple drug therapy except bloating.
In H. pylori-positive patients, the addition of probiotic L. reuteri to standard triple drug therapy was linked with substantial improvements in H. pylori elimination rate, and reduction in the intensity of gastrointestinal symptoms and treatment-linked adverse effects.
Journal of Pharmaceutical Research International
Efficacy of Lactobacillus reuteri Supplementation in Eradication of H. pylori: A Comparison Study with Triple Drug Therapy
Kumar Parth et al.
Comments (0)