Categories
Change Password!
Reset Password!


In a randomized trial, introducing peanuts in infancy and continuing until age 5 markedly reduced the chances of developing peanut allergy.
Peanut consumption from infancy to age 5 substantially reduces peanut allergy prevalence, with tolerance persisting into adolescence.
In a randomized trial, introducing peanuts in infancy and continuing until age 5 markedly reduced the chances of developing peanut allergy. A subsequent extension of the trial confirmed that this protective effect persisted even after a year of peanut avoidance. The goal of this follow-up study was to determine the long-term durability of peanut tolerance at 144 months (12 years), following years of unrestricted peanut consumption or avoidance.
Volunteers from the original peanut consumption trial were scrutinized for peanut allergy after an extended period of consuming or abstaining from peanuts as preferred. The prevalence of peanut allergy at age 144 months was the key outcome ascertained.
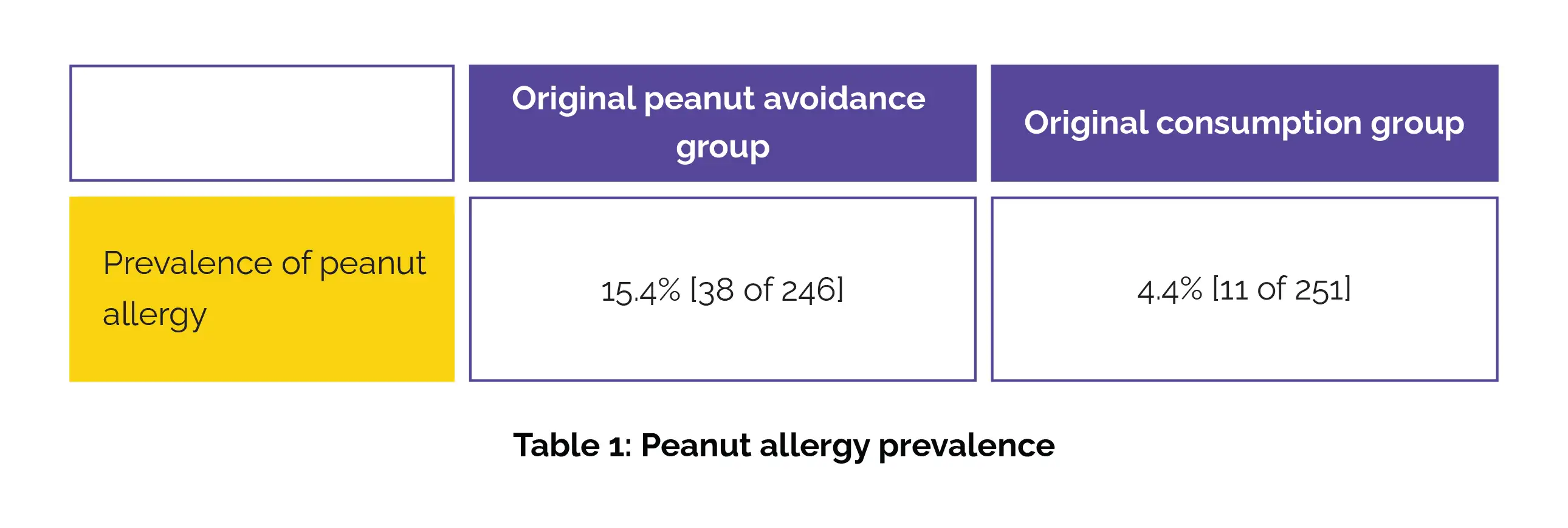
Of the initial 640 volunteers, 508 (79.4%) were enrolled, with 497 offering complete primary outcome data. At 144 months, the prevalence of peanut allergy remained quite higher in the original peanut avoidance group as opposed to the original consumption group (Table 1).
Between 72 and 144 months, both groups reported prolonged periods of peanut avoidance. At 144 months, the peanut consumption group illustrated Ara h2-specific IgE (serologic marker for peanut allergy) levels of 0.03 ± 3.42 kU/L and peanut-specific IgG4 levels of 535.5 ± 4.98 μg/L, when compared to 0.06 ± 11.21 kU/L and 209.3 ± 3.84 μg/L, respectively, in the peanut avoidance group. Adverse events were rare and primarily linked with food challenges.
Early peanut intake, maintained until age 5, led to sustained tolerance into adolescence, regardless of subsequent peanut consumption habits. This underscores the potential for long-term prevention and tolerance in food allergy management.
NEJM Evidence
Follow-up to Adolescence after Early Peanut Introduction for Allergy Prevention
George Du Toit et al.
Comments (0)