Categories
Change Password!
Reset Password!


An evaluation of the impact of Etoricoxib on taxane-associated acute pain syndrome (T-APS) was the objective of this phase II randomized study.
In women with breast cancer, prophylactic usage of 60 mg Etoricoxib has the potential to attenuate Docetaxel-elicited peripheral neuropathy and the severity and incidence of Docetaxel-triggered acute pain syndrome.
An evaluation of the impact of Etoricoxib on taxane-associated acute pain syndrome (T-APS) was the objective of this phase II randomized study.
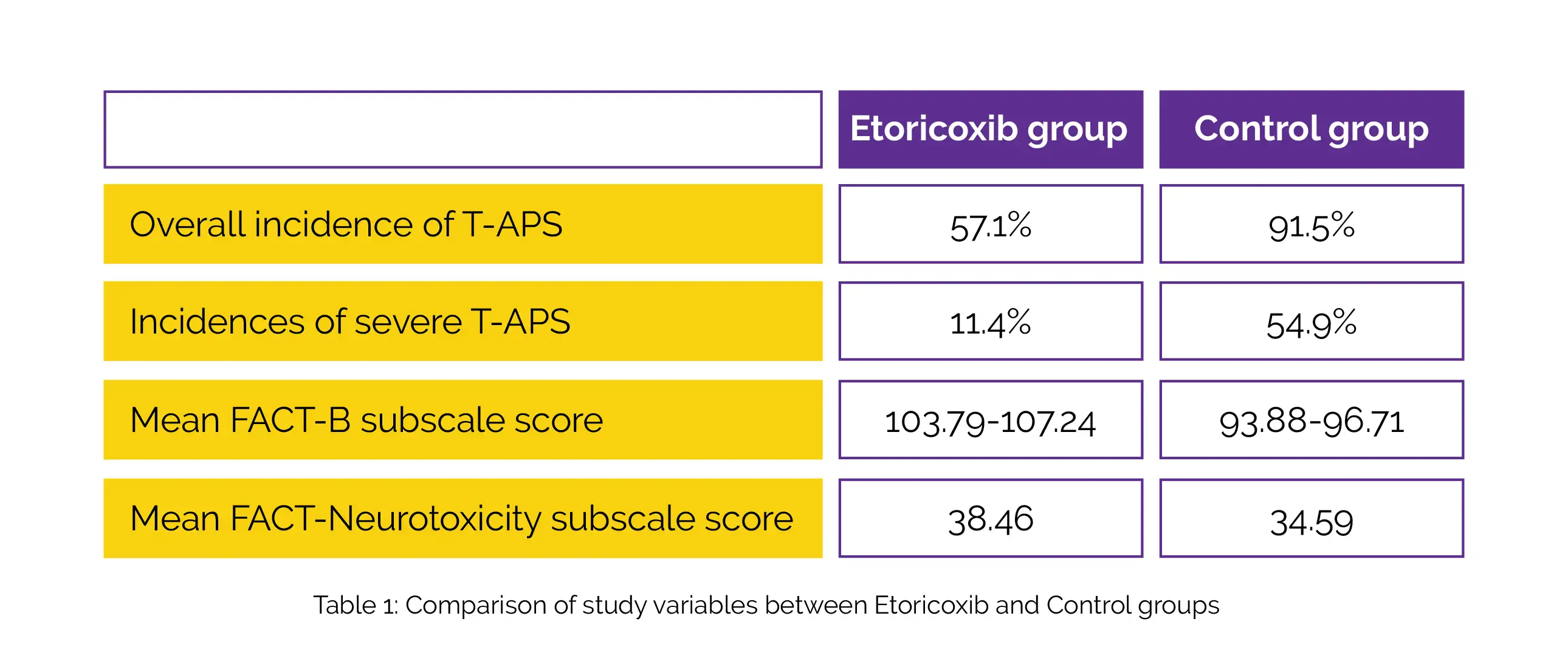
Four cycles of chemotherapy based on Docetaxel were given to a total of 144 breast cancer patients. Prophylactic Etoricoxib (60 mg, Day 1 to Day 8) and no prophylactic therapy were given to patients randomly assigned in a 1:1 ratio. The overall occurrence of T-APS across all cycles was the major outcome ascertained. The adverse events, chronic sensory and motor neurotoxicity, the Functional Assessment of Cancer Therapy–Breast (FACT-B) subscale, T-APS duration and severity, and occurrence of severe pain (defined as higher than 5 on a scale of 0–10) were considered the secondary outcomes.
Table 1 illustrated overall occurrence of T-APS across all chemotherapy cycles, incidences of severe T-APS, and mean FACT-B subscale score. The mean FACT-Neurotoxicity subscale score following four cycles of Docetaxel chemotherapy was greater in the Etoricoxib group when compared to the control group.
Contrasted to the control group, most peripheral sensory nerves in the Etoricoxib group had profoundly better conduction velocities and action potential amplitudes, according to electromyography.
Hence, Etoricoxib use was associated with prevention of T-APS in breast cancer people.
The European Journal of Cancer
Prevention of taxane-associated acute pain syndrome with Etoricoxib for patients with breast cancer: A phase II randomised trial
Junsheng Zhang et al.
Comments (0)