Categories
Change Password!
Reset Password!


This study sought to evaluate both the efficiency and safety of Abrocitinib monotherapy in those diagnosed with moderate-to-severe atopic dermatitis (AD), particularly those with allergic comorbidities.
Abrocitinib is safe, effective, and well-tolerated in patients having atopic dermatitis with or without allergic comorbidities (like rhinitis, allergic asthma, food allergy, conjunctivitis).
This study sought to evaluate both the efficiency and safety of Abrocitinib monotherapy in those diagnosed with moderate-to-severe atopic dermatitis (AD), particularly those with allergic comorbidities.
This post-hoc analysis utilized pooled data from three trials examining the efficacy of Abrocitinib when used as the sole treatment. These trials included one phase 2b and two phase 3 studies. The subjects in these trials were either eighteen years or older (phase 2b) or twelve years or older (phase 3) and had a confirmed diagnosis of AD. In the phase 2b trial, eligible participants were randomized in a 1:1:1:1:1 ratio to receive once-daily oral Abrocitinib at doses of 200, 100, 30, or 10 mg, or a placebo, once daily for twelve weeks.
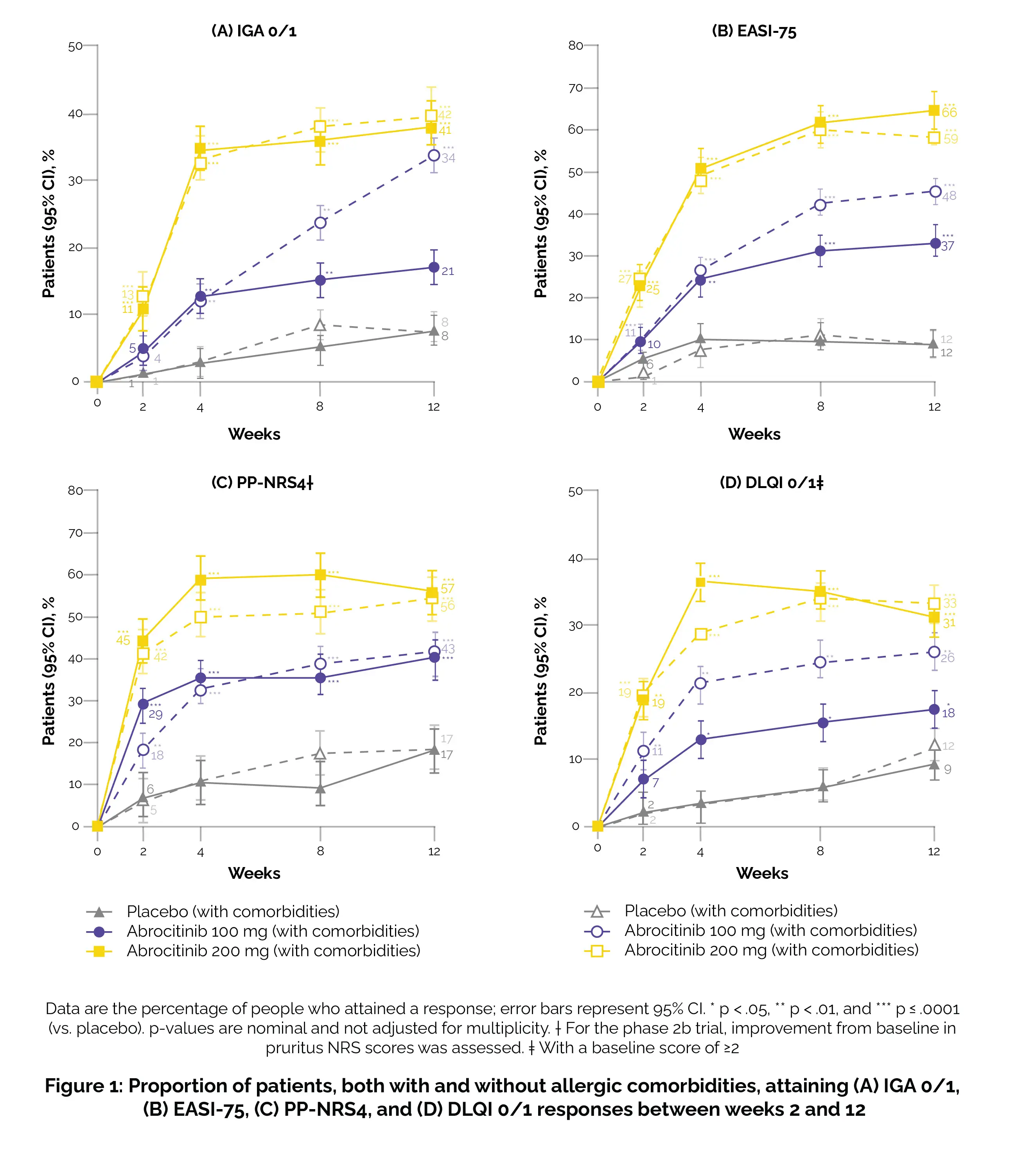
In the phase 3 trials, participants were randomly allocated in a 2:2:1 ratio to receive once-daily oral Abrocitinib at doses of 200 or 100 mg, or a placebo for 12 weeks. The evaluation was conducted on patients, both with and without allergic comorbidities (such as rhinitis, allergic asthma, food allergy, or conjunctivitis), regarding their response to the investigator's global assessment (IGA) criteria, aiming for a clear (0) or almost clear (1) status.
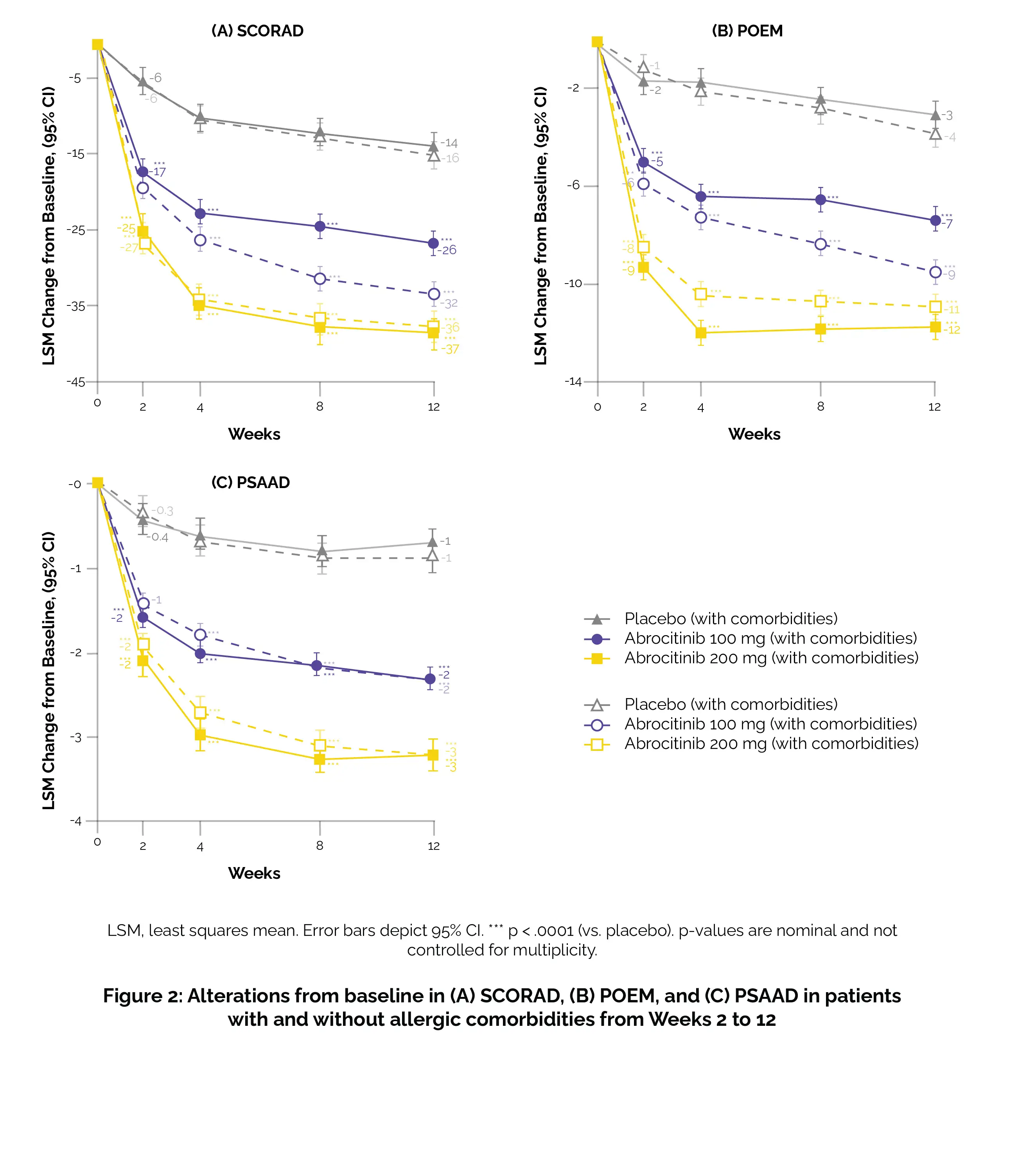
Additionally, scrutiny was applied for a ≥4-point improvement in the peak pruritus numerical rating scale (PP-NRS4), a ≥75% amelioration rate in the eczema area and severity index (EASI-75), and a response in the dermatology life quality index (DLQI) with a score of less than 2 when the baseline score was equal to or greater than 2. Additional results encompassed assessments using the SCORing Atopic Dermatitis (SCORAD), patient-oriented eczema measure (POEM), pruritus and symptoms assessment for atopic dermatitis (PSAAD), and the monitoring of treatment-emergent adverse events.
Out of the 942 patients, 498 (53%) reported having at least one allergic comorbidity, with 33% having asthma only, 17% having conjunctivitis only or rhinitis only or both, 15% having food allergies only, and 34% having more than one allergic comorbidity. Regardless of comorbidity status, during the period from week 2 to week 12, a higher percentage of patients treated with either Abrocitinib dose achieved outcomes such as EASI-75, PP-NRS4, DLQI 0/1, or IGA 0/1 compared to patients treated with a placebo (Figure 1).
Abrocitinib demonstrated larger changes from baseline in PSAAD, SCORAD, and POEM compared to placebo, irrespective of the presence or absence of allergic comorbidities (Figure 2).
Majority of treatment-emergent side effects were deemed as mild or moderate.
Abrocitinib is effective and well-received among patients dealing with moderate-to-severe AD, whether they have accompanying allergic comorbidities. Consequently, Abrocitinib appears to be a viable treatment choice for AD, independent of the existence of allergic comorbidities.
Allergy
Efficacy and safety of Abrocitinib in patients with moderate-to-severe atopic dermatitis and comorbid allergies
Peter Schmid-Grendelmeier et al.
Comments (0)