Categories
Change Password!
Reset Password!


This multicenter, randomized, double-blind trial (FAIR-HFpEF) sought to explore the clinical benefits of addressing iron insufficiency in those affected with heart failure with preserved ejection fraction (HFpEF).
In iron-deficient patients diagnosed with heart failure with preserved ejection fraction, treatment with ferric carboxymaltose is well-tolerated and improves exercise capacity.
This multicenter, randomized, double-blind trial (FAIR-HFpEF) sought to explore the clinical benefits of addressing iron insufficiency in those affected with heart failure with preserved ejection fraction (HFpEF).
In this study, intravenously administered ferric carboxymaltose (FCM) was compared with placebo (saline) in 200 individuals suffering from symptomatic HFpEF and iron inadequacy (serum ferritin less than 100 ng/mL or ferritin 100–299 ng/mL with transferrin saturation less than 20%). From baseline to week 24, the alteration in the 6-minute walk test distance (6MWTD) was the key endpoint ascertained. Alteration in health-related quality of life, patient global assessment, and New York Heart Association class were the secondary endpoints ascertained.
Due to delayed enrollment rate after 39 subjects were incorporated (median age 80 years, 62% women), the trial was halted. Volunteers allocated to FCM illustrated a greater improvement in 6MWTD from baseline to week 24 as opposed to those on placebo (least square mean difference 49 m), as shown in Figure 1:
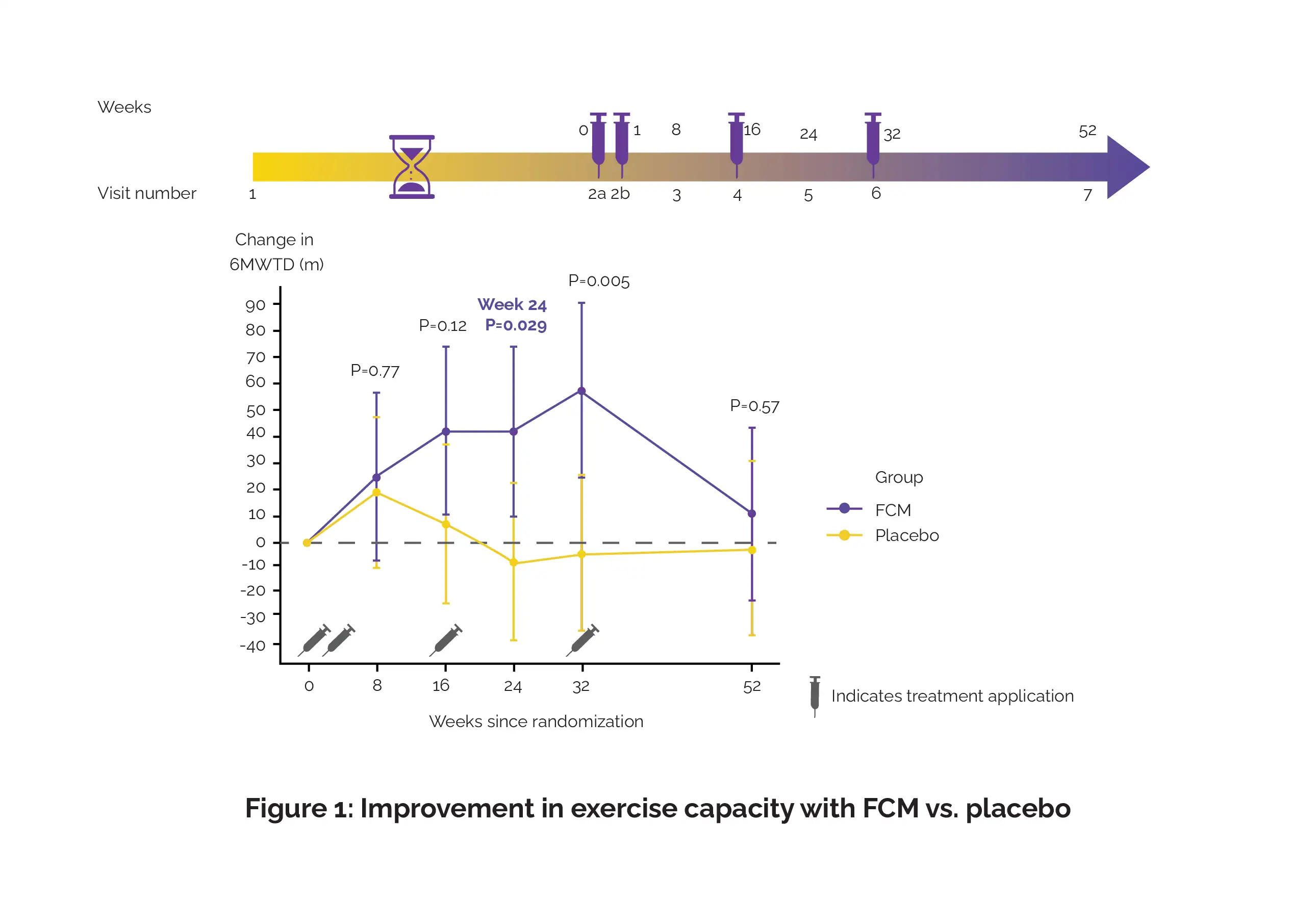
However, alterations in secondary endpoints were not markedly different between the study groups. When compared to placebo, FCM use was linked with fewer total adverse events (76 vs. 114) and severe adverse events (5 vs. 19; rate ratio 0.27).
Intravenously administered FCM boosted 6MWTD and was also linked with fewer severe adverse events. But, the trial was not well-powered to gauge the impact on symptoms or quality of life. Further investigation of intravenous iron in HFpEF patients with iron deficiency in a larger cohort is warranted.
European Heart Journal
Ferric carboxymaltose and exercise capacity in heart failure with preserved ejection fraction and iron deficiency: the FAIR-HFpEF trial
Stephan von Haehling et al.
Comments (0)