Categories
Change Password!
Reset Password!


The purpose of a randomized controlled trial was to assess the safety and effectiveness of Zinc supplementation to treat primary dysmenorrhea in young adolescent females.
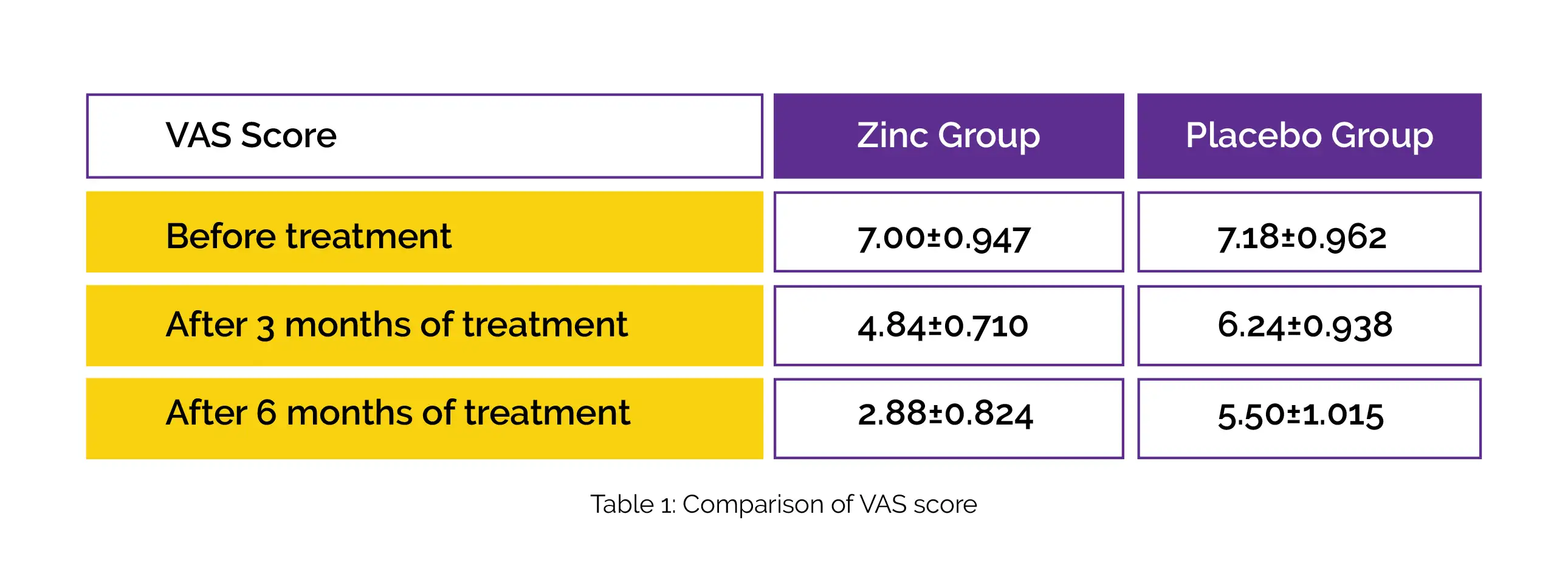
After three months of use, females who took Zinc supplements reported reduced pain compared to the placebo group, and a further decrease in pain intensity was noted after six months.
The purpose of a randomized controlled trial was to assess the safety and effectiveness of Zinc supplementation to treat primary dysmenorrhea in young adolescent females.
Through non-probability consecutive sampling, 100 patients with primary dysmenorrhea were included and divided into 2 groups randomly. For five days before and two days following the start of menstruation, patients in the intervention group (Group A) received 50 mg Zinc gluconate once a day, while patients in Group B were given a placebo. Utilizing visual analogue scale (VAS) for pain, the severity of primary dysmenorrhea was evaluated and contrasted in both groups after 3 and 6 months of therapy. The side effect profile was also determined.
In comparison to the placebo, Zinc gluconate therapy decreased the mean pain score in females with primary dysmenorrhea after three months, and the reduction continued after six months, as shown in Table 1:
Only a small percentage of subjects (4% after 3 months and 6% after 6 months) were affected by the adverse effects of Zinc supplementation.
Zinc gluconate 50 mg for 5 days prior to and 2 days following the start of menstruation is effective to treat primary dysmenorrhea. At this dosage and usage time, the medication's side effects are quite minimal.
Journal of Rawalpindi Medical College
Comparison of efficacy and safety of Zinc gluconate versus placebo for treatment of Primary Dysmenorrhea
Faiza Safdar et al.
Comments (0)