Categories
Change Password!
Reset Password!


An open-label, 16-week study assessed the safety and pharmacokinetics of nemolizumab (a novel monoclonal antibody that targets receptor alpha of interleukin-31 [IL-31]) for atopic dermatitis. The association between nemolizumab concentrations and clinical efficacy (pharmacokinetic/pharmacodynamic relation) and its impact on protein biomarkers were also investigated.
Nemolizumab therapy was found to reverse the pro-inflammatory biomarkers in the skin, signifying that the neuroimmune cytokine IL-31 is a crucial mediator of multiple pathways in adolescents (aged 12-17 years) with moderate to severe atopic dermatitis.
An open-label, 16-week study assessed the safety and pharmacokinetics of nemolizumab (a novel monoclonal antibody that targets receptor alpha of interleukin-31 [IL-31]) for atopic dermatitis. The association between nemolizumab concentrations and clinical efficacy (pharmacokinetic/pharmacodynamic relation) and its impact on protein biomarkers were also investigated.
The study recruited 20 people suffering from atopic dermatitis and related pruritus with the baseline average daily peak pruritus numeric rating scale (PP-NRS) intensity of at least 4. Subcutaneous administration of nemolizumab was given as a loading dose of 60 mg at the baseline.
This was followed by 30 mg every four weeks until the 12th week with background topical calcineurin inhibitors or topical corticosteroids. Participants were followed for eight more weeks. For biomarker evaluation, collection of stratum corneum and plasma samples was done.
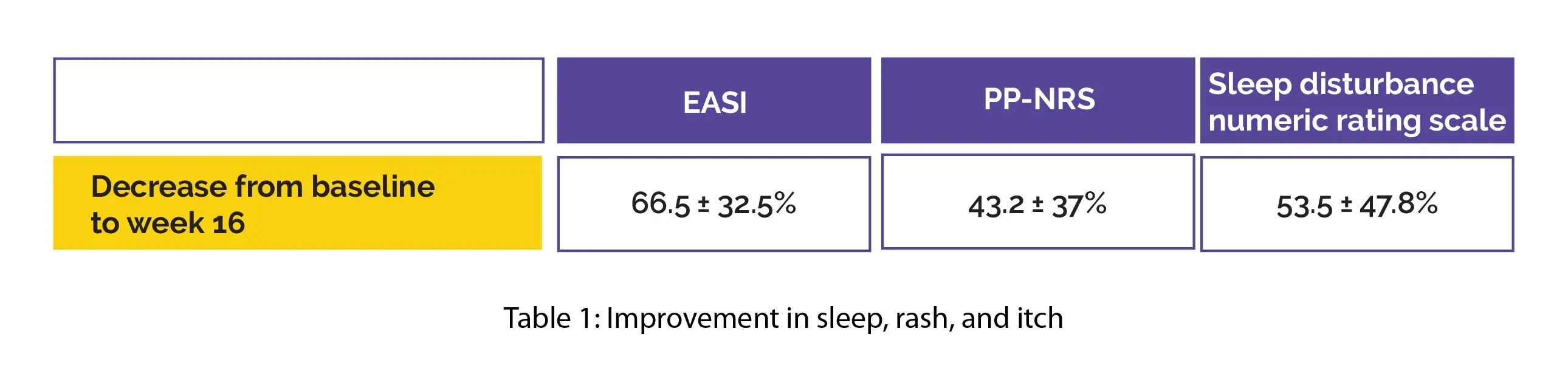
Nemolizumab's pharmacokinetics revealed a mean half-life of 16.7 ± 4.1 days, linear elimination, and a first-order absorption. Over the 16-week therapeutic period, the mean trough concentrations were found to range from 2935 ± 1029 ng/mL to 3292 ± 2018 ng/mL. A significant betterment was reported in sleep, rash, and itch with a reduction from baseline to week 16 in Eczema Area and Severity Index (EASI), PP-NRS, and in sleep disturbance numeric rating scale, as shown in Table 1:
As per the clinical efficacy assessment, the model-predicted exposure and effectiveness of nemolizumab were comparable in adolescents in comparison with adults receiving the same dosing schedule. Age did not influence pharmacokinetics parameters. Body weight was the principal source of pharmacokinetics variability.
Assessment of stratum corneum samples detected atopic dermatitis-linked pro-inflammatory biomarkers that were downregulated in clinical responders to nemolizumab (on the basis of EASI75 and PP-NRS ≥ 4) and upregulated in the lesional skin (in comparison with non-lesional skin).
Notably, 4 biomarkers (VEGF, CCL27, CCL20, CCL22) had alterations that were 1.9-3.5-fold greater in EASI responders when compared to EASI non-responders. There was no profound correlation between clinical scores and plasma biomarkers. The side effects were reported by 33.3% of people (n = 6) and were principally moderate or mild in severity.
Nemolizumab safety and pharmacokinetics profiles in atopic dermatitis adolescents showed consistency with prior nemolizumab studies in adults. Pharmacokinetics/pharmacodynamics models showed comparable exposure-response profiles in 12- to 17-year-old adolescents and adults for PP-NRS, EASI, Investigator Global Assessment.
Dermatology and Therapy
Pharmacokinetics, Safety, Efficacy, and Biomarker Profiles During Nemolizumab Treatment of Atopic Dermatitis in Adolescents
Robert Sidbury et al.
Comments (0)