Categories
Change Password!
Reset Password!


This single-center, open-label, randomized controlled trial investigated the efficacy of oral Sildenafil versus Bosentan as monotherapies for pulmonary hypertension of the newborn (PPHN).
While both Sildenafil and Bosentan are well-tolerated in neonates with PPHN, Sildenafil achieves faster PASP and FiO2 reduction and is less likely to require additional vasodilators.
This single-center, open-label, randomized controlled trial investigated the efficacy of oral Sildenafil versus Bosentan as monotherapies for pulmonary hypertension of the newborn (PPHN).
The study enrolled term and late preterm neonates (gestational age≥ 34 weeks) diagnosed with PPHN, characterized by a pulmonary arterial systolic pressure (PASP) of more than 35 mmHg and requiring fraction of inspired oxygen (FiO2) of more than 0.21. Volunteers were randomly allocated to receive either oral Sildenafil or Bosentan. The key endpoint ascertained was a 25% decrease in PASP within 48 hours of treatment initiation.
A total of 36 neonates (18 in each group) were analyzed. Initial PASP levels were comparable between the study groups. The median (interquartile range) time to achieve the primary outcome—a 25% reduction in PASP within 48 hours—was markedly shorter in the Sildenafil group when compared to the Bosentan group (Table 1).
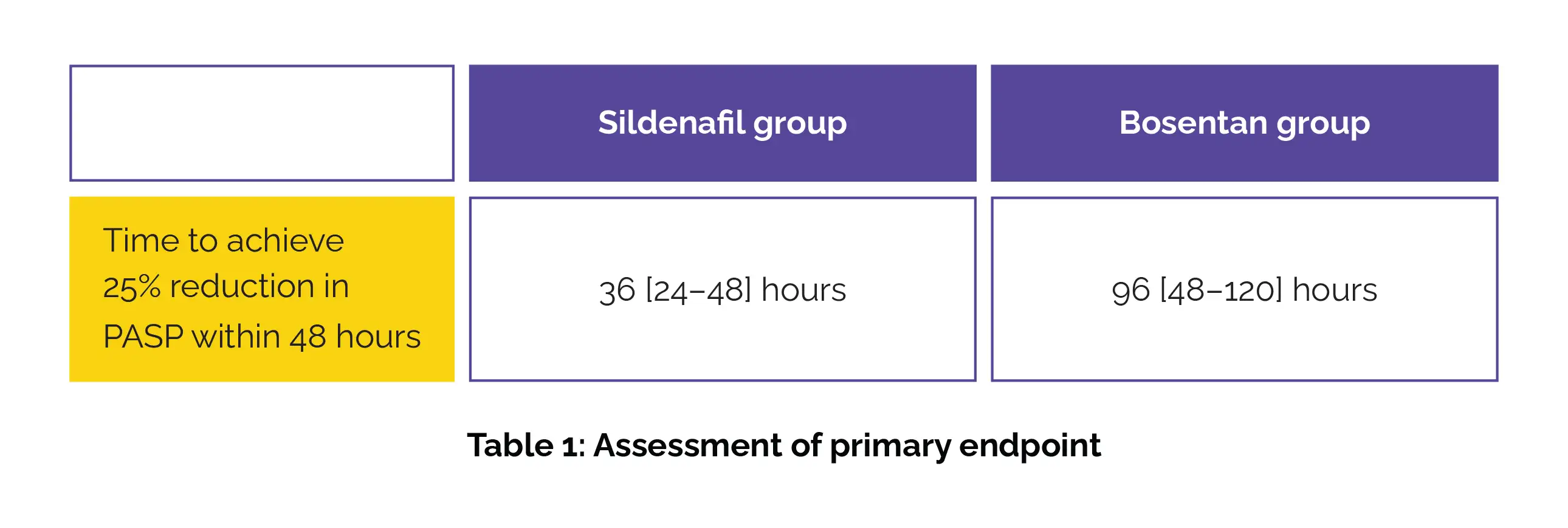
Furthermore, the Bosentan group required more frequent addition of other pulmonary vasodilators than Sildenafil group.
In neonates with PPHN, Sildenafil (phosphodiesterase-5 inhibitor) attained faster reductions in PASP and FiO2 when compared to Bosentan (endothelin-1 receptor antagonist). More multicenter blinded trials are needed to clarify Bosentan's utility relative to other pulmonary vasodilators, especially in resource-limited settings without inhaled nitric oxide.
BMC Pediatrics
Oral sildenafil versus bosentan for treatment of persistent pulmonary hypertension of the newborn: a randomized controlled trial
Aditya Kallimath et al.
Comments (0)