Categories
Change Password!
Reset Password!


This post-hoc analysis sought to assess the prolonged effectiveness of continuous and decreased-dose Abrocitinib, withdrawal, and therapy reintroduction reaction after flare in JAK1 Atopic Dermatitis Efficacy and Safety (JADE) REGIMEN participants.
Abrocitinib [Janus kinase (JAK) inhibitor], whether through varied dosing strategies, proves effective and safe in enhancing the quality of life for both adults and adolescents with moderate-to-severe atopic dermatitis.
This post-hoc analysis sought to assess the prolonged effectiveness of continuous and decreased-dose Abrocitinib, withdrawal, and therapy reintroduction reaction after flare in JAK1 Atopic Dermatitis Efficacy and Safety (JADE) REGIMEN participants.
Individuals (aged 12 to 17 years) and adults suffe atopic dermatitis (AD) responding to Abrocitinib 200 mg induction were picked. They were allocated to 40 weeks’ maintenance using placebo or Abrocitinib (200 mg/100 mg). Those encountering a flare-up or itch during maintenance obtained the rescue therapy.
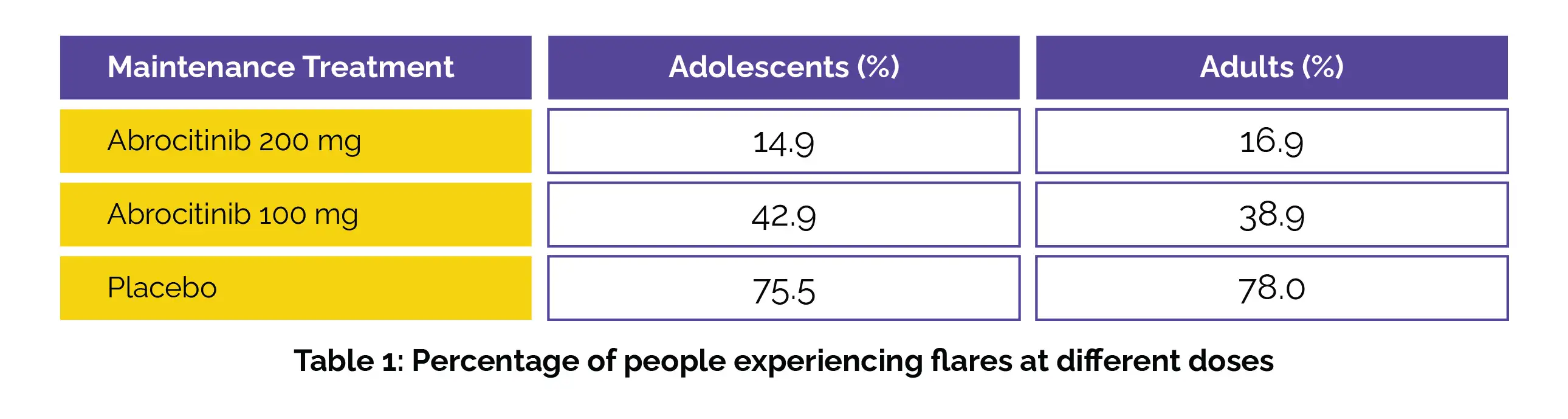
Out of 246 adolescents, 145 (58.9%), and among 981 adults, 655 (66.8%) responded to the induction. They experienced comparable proportions of flare during maintenance with Abrocitinib and placebo, as shown in the following Table 1:
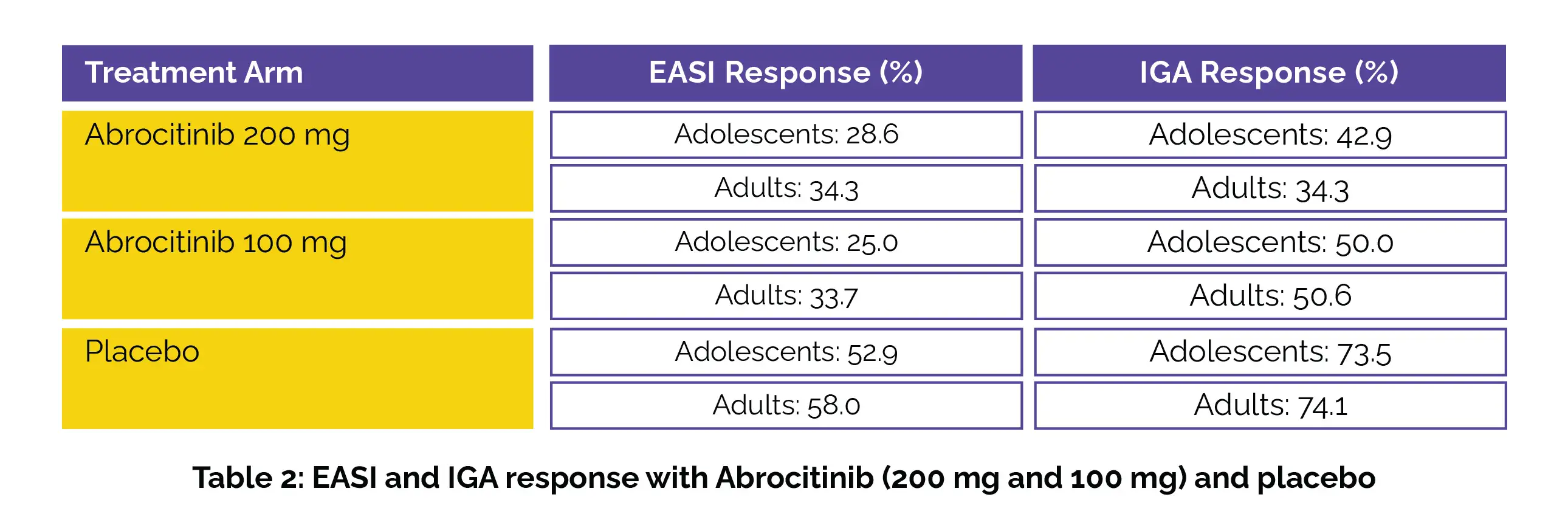
The percentage of participants experiencing the Eczema Area and Severity Index (EASI) response and the Investigator's Global Assessment (IGA) response is illustrated in the following Table 2:
Abrocitinib showed consistent safety across ages, with higher nausea incidence in adolescents.
Abrocitinib therapy is useful for avoiding flare-ups in AD-affected adolescents and adults.
Journal of Dermatological Treatment
Efficacy and safety of Abrocitinib monotherapy in adolescents and adults: a post hoc analysis of the phase 3 JAK1 atopic dermatitis efficacy and safety (JADE) REGIMEN clinical trial
Carsten Flohr et al.
Comments (0)