Categories
Change Password!
Reset Password!


Treatment with lidocaine medicated plaster effectively reduces pain intensity in people with post-herpetic neuralgia.
A retrospective observational investigation added further weight to the growing body of research and clinical evidence that 700 mg lidocaine medicated plaster therapy is effective and shows good tolerability for the management of postherpetic neuralgia. This study was carried out to report the follow-up of 31 people suffering from postherpetic neuralgia (a neuropathic pain syndrome following herpes zoster infection) and treated with 700 mg lidocaine medicated plaster, focusing on safety, efficacy, and quality of life.
Participants were regularly followed for co-analgesic intake, pain intensity, side effects, quality of life utilizing the European Quality of Life Five Dimension (EQ-5D), and patient satisfaction for eight weeks. At recruitment, 18 people (58.1%) were managed with at least 1 postherpetic neuralgia concomitant medication, for which the dosing and number remained constant during the study. A decrease in mean average pain intensity was reported at week 4 and week 8 when compared to baseline, as shown in Table 1:
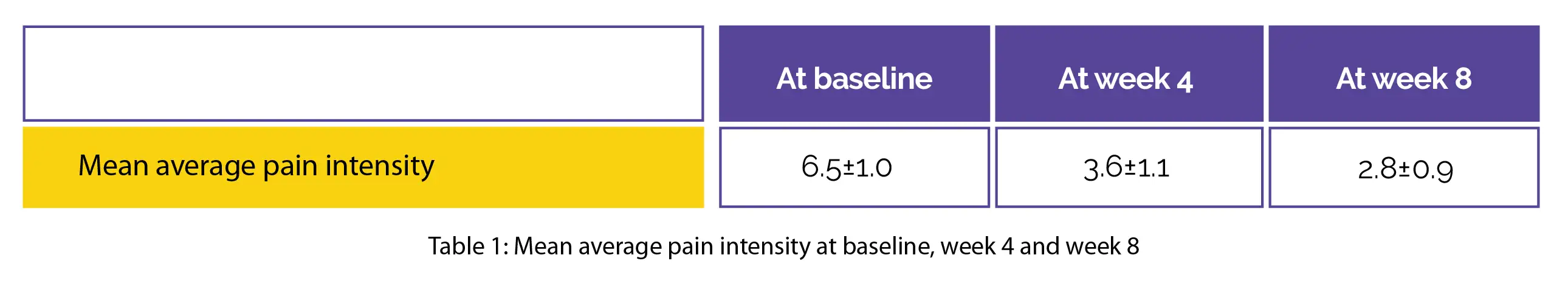
A total of 4 people reported erythema, and 1 complained of vesicles eruption linked with pruritus. The EQ-5D at weeks 4 and 8 of therapy illustrated long-lasting improvements in all the domains except for the "depression/anxiety" domain. At week 8, <80% of people reported to be very satisfied or satisfied.
Thus, 700 mg lidocaine medicated plaster therapy is well-tolerated and yields satisfactory outcomes in individuals diagnosed with post-herpetic neuralgia.
The European Review for Medical and Pharmacological Sciences
Lidocaine 700 mg medicated plaster for post-herpetic neuralgia: focus on Quality of Life, effectiveness and safety - a retrospective observational study
L G Giaccari et al.
Comments (0)