Categories
Change Password!
Reset Password!


Oral immunotherapy (including probiotic and peanut oral immunotherapy) helps to significantly increase peanut ingestion tolerance.
A recent follow-up study of a peanut oral immunotherapy trial, PPOIT-003 published in “Allergy” investigated the long-term benefits and risks of different clinical outcomes like desensitization, remission, and persistent allergy. Peanut allergy-affected children (aged 1-10 years) from the PPOIT-003 trial who completed their treatment were tracked for an additional 2 years in the PPOIT-003LT study. Researchers monitored peanut ingestion, allergic reactions, and health-related quality of life (HRQOL).
Outcomes were examined across different clinical outcomes: remission, desensitization without remission (DWR), and allergic status. Peanut ingestion was similar for probiotic and peanut oral immunotherapy (PPOIT) and oral immunotherapy (OIT) groups at 2 years, both higher than placebo. Reactions decreased over time across all groups and outcomes, as depicted in Table 1 below.
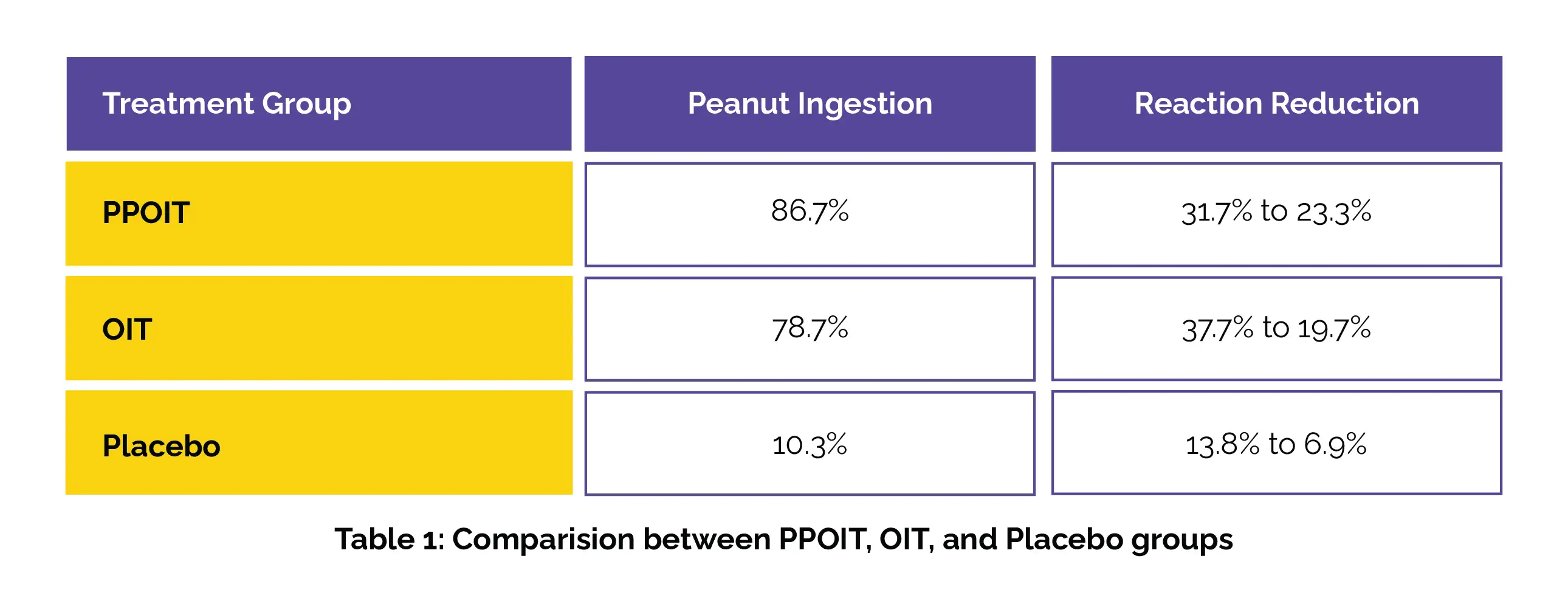
At 2 years after treatment, remission and allergic groups exhibited comparable reaction rates (Risk difference [RD] 0.09, 95% CI −0.03 to 0.20, p = .127), while DWR participants showed more reactions as opposed to remission (RD −0.21, 95% CI −0.39 to −0.03, p = .02) and allergic groups (RD 0.30, 95% CI 0.13 to 0.47, p = .001). At 2 years, adrenaline injector use was reported by 0% of remission, 5.3% of DWR, and 2.3% of allergic participants. Additionally, remission participants also illustrated significantly greater improvement in HRQOL compared to DWR (Mean difference [MD] −0.54) and allergic (MD −0.82) participants.
This study confirmed that oral immunotherapy can be effective in managing peanut allergies, with remission being the most favorable long-term outcome. Remission led to fewer reactions, no need for rescue medication, and a better quality of life compared to DWR and remaining allergic.
Allergy
Two-year post-treatment outcomes following peanut oral immunotherapy in the Probiotic and Peanut Oral Immunotherapy-003 Long-Term (PPOIT-003LT) study
Paxton Loke et. al.
Comments (0)